Protein-engineered biomaterials: nanoscale mimics of the extracellular matrix
- PMID: 20647034
- PMCID: PMC3033985
- DOI: 10.1016/j.bbagen.2010.07.005
Protein-engineered biomaterials: nanoscale mimics of the extracellular matrix
Abstract
Background: Traditional materials used as in vitro cell culture substrates are rigid and flat surfaces that lack the exquisite nano- and micro-scale features of the in vivo extracellular environment. While these surfaces can be coated with harvested extracellular matrix (ECM) proteins to partially recapitulate the bio-instructive nature of the ECM, these harvested proteins often exhibit large batch-to-batch variability and can be difficult to customize for specific biological studies. In contrast, recombinant protein technology can be utilized to synthesize families of 3 dimensional protein-engineered biomaterials that are cyto-compatible, reproducible, and fully customizable.
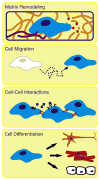
Scope of review: Here we describe a modular design strategy to synthesize protein-engineered biomaterials that fuse together multiple repeats of nanoscale peptide design motifs into full-length engineered ECM mimics.
Major conclusions: Due to the molecular-level precision of recombinant protein synthesis, these biomaterials can be tailored to include a variety of bio-instructional ligands at specified densities, to exhibit mechanical properties that match those of native tissue, and to include proteolytic target sites that enable cell-triggered scaffold remodeling. Furthermore, these biomaterials can be processed into forms that are injectable for minimally-invasive delivery or spatially patterned to enable the release of multiple drugs with distinct release kinetics.
General significance: Given the reproducibility and flexibility of these protein-engineered biomaterials, they are ideal substrates for reductionist biological studies of cell-matrix interactions, for in vitro models of physiological processes, and for bio-instructive scaffolds in regenerative medicine therapies. This article is part of a Special Issue entitled Nanotechnologies - Emerging Applications in Biomedicine.
2010 Elsevier B.V. All rights reserved.
Figures






References
-
- Park H, Cannizzaro C, Vunjak-Novakovic G, Langer R, Vacanti CA, Farokhzad OC. Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Eng. 2007;13:1867–1877. - PubMed
-
- Szu-Yuan C, Chao-Min C, LeDuc PR. Composite polymer systems with control of local substrate elasticity and their effect on cytoskeletal and morphological characteristics of adherent cells. Biomaterials. 2009;30:3136–3142. - PubMed
-
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
-
- Chen CS. Mechanotransduction - a field pulling together? J Cell Sci. 2008;121:3285–3292. - PubMed
-
- Wong Po Foo C, Heilshorn SC. Protein-engineered biomaterials. In: Park SJ, Cochran JR, editors. Protein Engineering and Design. CRC Press; Boca Raton: 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

