SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity
- PMID: 20647045
- PMCID: PMC2943385
- DOI: 10.1016/j.freeradbiomed.2010.07.009
SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity
Abstract
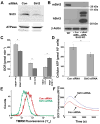
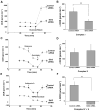
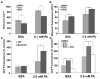
SIRT3 is the primary mitochondrial deacetylase that modulates mitochondrial metabolic and oxidative stress regulatory pathways. However, its role in response to nutrient excess remains unknown. Thus, we investigated SIRT3 regulation of the electron transfer chain and evaluated the role of SIRT3 in hepatic lipotoxic stress. SIRT3-depleted HepG2 cells show diffuse disruption in mitochondrial electron transfer chain functioning, a concurrent reduction in the mitochondrial membrane potential, and excess basal reactive oxygen species levels. As this phenotype may predispose to increased lipotoxic hepatic susceptibility we evaluated the expression of SIRT3 in murine liver after chronic high-fat feeding. In this nutrient-excess model SIRT3 transcript and protein levels are downregulated in parallel with increased hepatic fat storage and oxidative stress. Palmitate was used to investigate lipotoxic susceptibility in SIRT3 knockout mouse primary hepatocytes and SIRT3-siRNA-transfected HepG2 cells. Under SIRT3-deficient conditions palmitate enhances reactive oxygen species and increases hepatocyte death. Reconstitution of SIRT3 levels and/or treatment with N-acetylcysteine ameliorates these adverse effects. In conclusion SIRT3 functions to ameliorate hepatic lipotoxicity, although paradoxically, exposure to high fat downregulates this adaptive program in the liver. This SIRT3-dependent lipotoxic susceptibility is possibly modulated, in part, by SIRT3-mediated control of electron transfer chain flux.
Published by Elsevier Inc.
Figures


References
-
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

