Dimethylarginine metabolism during acute and chronic rejection of rat renal allografts
- PMID: 20647192
- PMCID: PMC3006445
- DOI: 10.1093/ndt/gfq392
Dimethylarginine metabolism during acute and chronic rejection of rat renal allografts
Abstract
Background: Dimethylarginines are inhibitors of NO synthesis and are involved in the pathogenesis of vascular diseases. In this study, we ask the question if asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) levels change during fatal and reversible acute rejection, and contribute to the pathogenesis of chronic vasculopathy.
Methods: The Dark Agouti to Lewis rat strain combination was used to investigate fatal acute rejection. Fischer 344 kidneys were transplanted to Lewis rats to study reversible acute rejection episode and the process of chronic rejection. Isograft recipients and untreated Lewis rats were used as controls. l-arginine derivatives were determined by HPLC, and ADMA-metabolizing enzymes were studied by quantitative RT-PCR and western blotting.
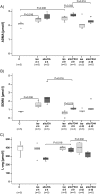
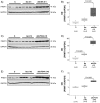
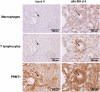
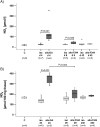
Results: Renal transplantation transiently increased dimethylarginine levels independent of acute rejection. ADMA plasma levels did not importantly differ between recipients undergoing fatal or reversible acute rejection, whereas SDMA was even lower in recipients of Fisher 344 grafts. In comparison to isograft recipients, ADMA and SDMA levels were slightly elevated during reversible, but not during the process of chronic rejection. Increased dimethylarginine levels, however, did not block NO synthesis. Interestingly, protein methylation, but not ADMA degradation, was increased in allografts.
Conclusions: Our data do not support the concept that renal allografts are protected from fatal rejection by dimethylarginines. Dimethylarginines may play a role in triggering chronic rejection, but a contribution to vascular remodelling itself is improbable. In contrast, differential arginine methylation of yet unknown proteins by PRMT1 may be involved in the pathogenesis of acute and chronic rejection.
Figures


Similar articles
-
Increased symmetrical dimethylarginine in ischemic acute kidney injury as a causative factor of renal L-arginine deficiency.Transl Res. 2013 Aug;162(2):67-76. doi: 10.1016/j.trsl.2013.04.005. Epub 2013 May 22. Transl Res. 2013. PMID: 23707198
-
Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): the ADMA, SDMA and hArg paradoxes.Cardiovasc Diabetol. 2018 Jan 4;17(1):1. doi: 10.1186/s12933-017-0656-x. Cardiovasc Diabetol. 2018. PMID: 29301528 Free PMC article.
-
Severely decreased activity of placental dimethylarginine dimethylaminohydrolase in pre-eclampsia.Eur J Obstet Gynecol Reprod Biol. 2012 Apr;161(2):152-6. doi: 10.1016/j.ejogrb.2011.12.032. Epub 2012 Jan 28. Eur J Obstet Gynecol Reprod Biol. 2012. PMID: 22285683
-
Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA).Toxins (Basel). 2017 Mar 6;9(3):92. doi: 10.3390/toxins9030092. Toxins (Basel). 2017. PMID: 28272322 Free PMC article. Review.
-
Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach.Int J Mol Sci. 2019 Jul 26;20(15):3668. doi: 10.3390/ijms20153668. Int J Mol Sci. 2019. PMID: 31357472 Free PMC article. Review.
Cited by
-
Expression of acetylcholine receptors by experimental rat renal allografts.Biomed Res Int. 2014;2014:289656. doi: 10.1155/2014/289656. Epub 2014 Jul 9. Biomed Res Int. 2014. PMID: 25121092 Free PMC article.
-
Uremic Toxins, Oxidative Stress, Atherosclerosis in Chronic Kidney Disease, and Kidney Transplantation.Oxid Med Cell Longev. 2021 Feb 11;2021:6651367. doi: 10.1155/2021/6651367. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628373 Free PMC article. Review.
-
Monocytic Tissue Transglutaminase in a Rat Model for Reversible Acute Rejection and Chronic Renal Allograft Injury.Mediators Inflamm. 2015;2015:429653. doi: 10.1155/2015/429653. Epub 2015 Apr 30. Mediators Inflamm. 2015. PMID: 26063971 Free PMC article.
-
Protein arginine methyltransferases in renal development, injury, repair, and fibrosis.Front Pharmacol. 2023 Feb 3;14:1123415. doi: 10.3389/fphar.2023.1123415. eCollection 2023. Front Pharmacol. 2023. PMID: 36817133 Free PMC article. Review.
-
Protein arginine methyltransferase 1 promoted the growth and migration of cancer cells in esophageal squamous cell carcinoma.Tumour Biol. 2016 Feb;37(2):2613-9. doi: 10.1007/s13277-015-4098-3. Epub 2015 Sep 22. Tumour Biol. 2016. PMID: 26392112
References
-
- Wilcken DE, Sim AS, Wang J, et al. Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of l-arginine on its metabolism. Mol Genet Metab. 2007;91:309–317. - PubMed
-
- Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Ren Physiol. 2008;294:F1–F9. - PubMed
-
- Boger RH, Zoccali C. ADMA: a novel risk factor that explains excess cardiovascular event rate in patients with end-stage renal disease. Atheroscler Suppl. 2003;4:23–28. - PubMed
-
- Boger RH, Cooke JP, Vallance P. ADMA: an emerging cardiovascular risk factor. Vasc Med. 2005;10:S1–S2. - PubMed
-
- Boger RH, Bode-Boger SM, Tsao PS, et al. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol. 2000;36:2287–2295. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

