Rituximab versus cyclophosphamide for ANCA-associated vasculitis
- PMID: 20647199
- PMCID: PMC3137658
- DOI: 10.1056/NEJMoa0909905
Rituximab versus cyclophosphamide for ANCA-associated vasculitis
Abstract
Background: Cyclophosphamide and glucocorticoids have been the cornerstone of remission-induction therapy for severe antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis for 40 years. Uncontrolled studies suggest that rituximab is effective and may be safer than a cyclophosphamide-based regimen.
Methods: We conducted a multicenter, randomized, double-blind, double-dummy, noninferiority trial of rituximab (375 mg per square meter of body-surface area per week for 4 weeks) as compared with cyclophosphamide (2 mg per kilogram of body weight per day) for remission induction. Glucocorticoids were tapered off; the primary end point was remission of disease without the use of prednisone at 6 months.
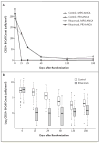
Results: Nine centers enrolled 197 ANCA-positive patients with either Wegener's granulomatosis or microscopic polyangiitis. Baseline disease activity, organ involvement, and the proportion of patients with relapsing disease were similar in the two treatment groups. Sixty-three patients in the rituximab group (64%) reached the primary end point, as compared with 52 patients in the control group (53%), a result that met the criterion for noninferiority (P<0.001). The rituximab-based regimen was more efficacious than the cyclophosphamide-based regimen for inducing remission of relapsing disease; 34 of 51 patients in the rituximab group (67%) as compared with 21 of 50 patients in the control group (42%) reached the primary end point (P=0.01). Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. There were no significant differences between the treatment groups with respect to rates of adverse events.
Conclusions: Rituximab therapy was not inferior to daily cyclophosphamide treatment for induction of remission in severe ANCA-associated vasculitis and may be superior in relapsing disease. (Funded by the National Institutes of Allergy and Infectious Diseases, Genentech, and Biogen; ClinicalTrials.gov number, NCT00104299.)
2010 Massachusetts Medical Society
Figures

Comment in
-
Rituximab in ANCA-associated disease.N Engl J Med. 2010 Jul 15;363(3):285-6. doi: 10.1056/NEJMe1004992. N Engl J Med. 2010. PMID: 20647204 No abstract available.
-
Is rituximab superior to cyclophosphamide for ANCA-associated vasculitis for induction of remission, and with a better safety profile?Curr Rheumatol Rep. 2010 Dec;12(6):395-8. doi: 10.1007/s11926-010-0133-y. Curr Rheumatol Rep. 2010. PMID: 20844995 No abstract available.
-
Therapy: Rituximab has similar short-term safety and efficacy to cyclophosphamide in ANCA-associated vasculitis.Nat Rev Rheumatol. 2010 Oct;6(10):556. doi: 10.1038/nrrheum.2010.150. Nat Rev Rheumatol. 2010. PMID: 20925151 No abstract available.
-
Rituximab or cyclophosphamide in ANCA-associated renal vasculitis.N Engl J Med. 2010 Nov 18;363(21):2073; author reply 2073-4. doi: 10.1056/NEJMc1009101. N Engl J Med. 2010. PMID: 21083398 No abstract available.
-
Rituximab or cyclophosphamide in ANCA-associated renal vasculitis.N Engl J Med. 2010 Nov 18;363(21):2072-3; author reply 2073-4. doi: 10.1056/NEJMc1009101. N Engl J Med. 2010. PMID: 21083399 No abstract available.
-
Rituximab or cyclophosphamide in ANCA-associated renal vasculitis.N Engl J Med. 2010 Nov 18;363(21):2072; author reply 2073-4. doi: 10.1056/NEJMc1009101. N Engl J Med. 2010. PMID: 21083401 No abstract available.
References
-
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: the proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. - PubMed
-
- Finkielman JD, Lee AS, Hummel AM, et al. ANCA are detectable in nearly all patients with active severe Wegener’s granulomatosis. Am J Med. 2007;120(7):643.e9–643.14. - PubMed
-
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. - PubMed
-
- Guillevin L, Durand-Gasselin B, Cevallos R, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42:421–30. - PubMed
-
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum. 2000;43:1021–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- RR024150-01/RR/NCRR NIH HHS/United States
- K24 AR02224/AR/NIAMS NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- N01 AI015416/AI/NIAID NIH HHS/United States
- M01 RR000533/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- K24 AR002224/AR/NIAMS NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- RR 025771/RR/NCRR NIH HHS/United States
- UL1 RR024150/RR/NCRR NIH HHS/United States
- U01 AR051874/AR/NIAMS NIH HHS/United States
- K23 AR052820/AR/NIAMS NIH HHS/United States
- M01 RR00533/RR/NCRR NIH HHS/United States
- K24 AR049185/AR/NIAMS NIH HHS/United States
- RR025005/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
