Enhancement of procollagen biosynthesis by p180 through augmented ribosome association on the endoplasmic reticulum in response to stimulated secretion
- PMID: 20647306
- PMCID: PMC2943289
- DOI: 10.1074/jbc.M109.094607
Enhancement of procollagen biosynthesis by p180 through augmented ribosome association on the endoplasmic reticulum in response to stimulated secretion
Abstract
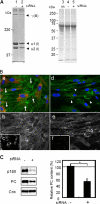
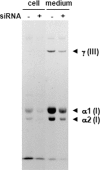
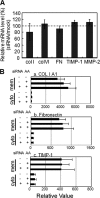
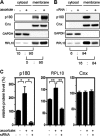
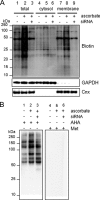
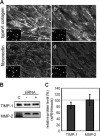
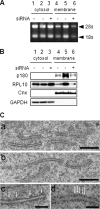
A coiled-coil microtubule-bundling protein, p180, was originally reported as a ribosome-binding protein on the rough endoplasmic reticulum (ER) and is highly expressed in secretory tissues. Recently, we reported a novel role for p180 in the trans-Golgi network (TGN) expansion following stimulated collagen secretion. Here, we show that p180 plays a key role in procollagen biosynthesis and secretion in diploid fibroblasts. Depletion of p180 caused marked reductions of secreted collagens without significant loss of the ER membrane or mRNA. Metabolic labeling experiments revealed that the procollagen biosynthetic activity was markedly affected following p180 depletion. Moreover, loss of p180 perturbs ascorbate-stimulated de novo biosynthesis mainly in the membrane fraction with a preferential secretion defect of large proteins. At the EM level, one of the most prominent morphological features of p180-depleted cells was insufficient ribosome association on the ER membranes. In contrast, the ER of control cells was studded with numerous ribosomes, which were further enhanced by ascorbate. Similarly biochemical analysis confirmed that levels of membrane-bound ribosomes were altered in a p180-dependent manner. Taken together, our data suggest that p180 plays crucial roles in enhancing collagen biosynthesis at the entry site of the secretory compartments by a novel mechanism that mainly involves facilitating ribosome association on the ER.
Figures

Similar articles
-
Expansion of the trans-Golgi network following activated collagen secretion is supported by a coiled-coil microtubule-bundling protein, p180, on the ER.Exp Cell Res. 2010 Feb 1;316(3):329-40. doi: 10.1016/j.yexcr.2009.11.009. Epub 2009 Nov 27. Exp Cell Res. 2010. PMID: 19932094
-
Regulation of polysome assembly on the endoplasmic reticulum by a coiled-coil protein, p180.Nucleic Acids Res. 2012 Apr;40(7):3006-17. doi: 10.1093/nar/gkr1197. Epub 2011 Dec 7. Nucleic Acids Res. 2012. PMID: 22156060 Free PMC article.
-
p180 promotes the ribosome-independent localization of a subset of mRNA to the endoplasmic reticulum.PLoS Biol. 2012;10(5):e1001336. doi: 10.1371/journal.pbio.1001336. Epub 2012 May 29. PLoS Biol. 2012. PMID: 22679391 Free PMC article.
-
Posttranslational events in collagen biosynthesis.Methods Cell Biol. 1981;23:119-36. doi: 10.1016/s0091-679x(08)61495-8. Methods Cell Biol. 1981. PMID: 7035799 Review. No abstract available.
-
ER to Golgi-Dependent Protein Secretion: The Conventional Pathway.Methods Mol Biol. 2016;1459:3-29. doi: 10.1007/978-1-4939-3804-9_1. Methods Mol Biol. 2016. PMID: 27665548 Review.
Cited by
-
Endoplasmic reticulum stress induces different molecular structural alterations in human dilated and ischemic cardiomyopathy.PLoS One. 2014 Sep 16;9(9):e107635. doi: 10.1371/journal.pone.0107635. eCollection 2014. PLoS One. 2014. PMID: 25226522 Free PMC article.
-
Stabilization of RRBP1 mRNA via an m6A-dependent manner in prostate cancer constitutes a therapeutic vulnerability amenable to small-peptide inhibition of METTL3.Cell Mol Life Sci. 2024 Oct 5;81(1):414. doi: 10.1007/s00018-024-05418-6. Cell Mol Life Sci. 2024. PMID: 39367907 Free PMC article.
-
Overexpression of ribosome binding protein 1 (RRBP1) in breast cancer.Clin Proteomics. 2012 Jun 18;9(1):7. doi: 10.1186/1559-0275-9-7. Clin Proteomics. 2012. PMID: 22709790 Free PMC article.
-
Ascorbate inducible N259 glycans on prolyl 4-hydroxylase subunit α1 promote hydroxylation and secretion of type I collagen.Cell Mol Life Sci. 2019 Sep;76(17):3449-3464. doi: 10.1007/s00018-019-03081-w. Epub 2019 Mar 27. Cell Mol Life Sci. 2019. PMID: 30919021 Free PMC article.
-
Component of splicing factor SF3b plays a key role in translational control of polyribosomes on the endoplasmic reticulum.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9340-9349. doi: 10.1073/pnas.1901742116. Epub 2019 Apr 19. Proc Natl Acad Sci U S A. 2019. PMID: 31004060 Free PMC article.
References
-
- Baumann O., Walz B. (2001) Int. Rev. Cytol. 205, 149–214 - PubMed
-
- Shibata Y., Voeltz G. K., Rapoport T. A. (2006) Cell 126, 435–439 - PubMed
-
- Canty E. G., Kadler K. E. (2005) J. Cell. Sci. 118, 1341–1353 - PubMed
-
- Myllyharju J. (2003) Matrix Biol. 22, 15–24 - PubMed
-
- Fromme J. C., Schekman R. (2005) Curr. Opin. Cell. Biol. 17, 345–352 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

