Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy
- PMID: 20647571
- PMCID: PMC2974595
- DOI: 10.1182/blood-2010-02-268466
Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy
Abstract
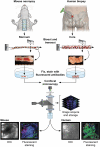
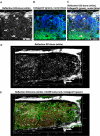
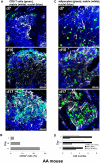
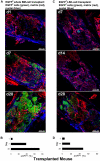
In many animals, blood cell production occurs in the bone marrow. Hematopoiesis is complex, requiring self-renewing and pluripotent stem cells, differentiated progenitor and precursor cells, and supportive stroma, adipose tissue, vascular structures, and extracellular matrix. Although imaging is a vital tool in hematology research, the 3-dimensional architecture of the bone marrow tissue in situ remains largely uncharacterized. The major hindrance to imaging the intact marrow is the surrounding bone structures are almost impossible to cut/image through. We have overcome these obstacles and describe a method whereby whole-mounts of bone marrow tissue were immunostained and imaged in 3 dimensions by confocal fluorescence and reflection microscopy. We have successfully mapped by multicolor immunofluorescence the localization pattern of as many as 4 cell features simultaneously over large tiled views and to depths of approximately 150 μm. Three-dimensional images can be assessed qualitatively and quantitatively to appreciate the distribution of cell types and their interrelationships, with minimal perturbations of the tissue. We demonstrate its application to normal mouse and human marrow, to murine models of marrow failure, and to patients with aplastic anemia, myeloid, and lymphoid cell malignancies. The technique should be generally adaptable for basic laboratory investigation and for clinical diagnosis of hematologic diseases.
Figures



References
-
- Choi JW, Pai SH, Choe HW. Significance of the ratio of reticulocyte subpopulations in bone marrow and peripheral blood from patients with myelodysplastic syndromes. Ann Hematol. 2003;82(4):259–261. - PubMed
-
- Abboud CN, Lichtman MA. Structure of the marrow and the hematopoietic microenvironment. In: Lichtman M, Beutler E, Kaushansky K, Kipps T, Seligsohn U, Prchal J, editors. Williams Hematology. 7th ed. New York: McGraw-Hill; 2006. pp. 35–72.
-
- Dao M, Verfaillie CM. Bone marrow microenvironment. In: Hoffman R, Benz EJ Jr, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, editors. Hematology: Basic Principles and Practice. 5th ed. New York: Churchill Livingstone; 2005. pp. 215–231.
-
- Lanotte M, Allen TD, Dexter TM. Histochemical and ultrastructural characteristics of a cell line from human bone-marrow stroma. J Cell Sci. 1981;50:281–297. - PubMed
-
- Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nature Immunol. 2006;7(4):333–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

