Hypothetical and factual willingness to participate in biobank research
- PMID: 20648060
- PMCID: PMC2987483
- DOI: 10.1038/ejhg.2010.106
Hypothetical and factual willingness to participate in biobank research
Abstract
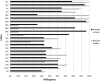
In the debate on biobank regulation, arguments often draw upon findings in surveys on public attitudes. However, surveys on willingness to participate in research may not always predict actual participation rates. We compared hypothetical willingness as estimated in 11 surveys conducted in Sweden, Iceland, United Kingdom, Ireland, United States and Singapore to factual participation rates in 12 biobank studies. Studies were matched by country and approximate time frame. Of 22 pairwise comparisons, 12 suggest that factual willingness to participate in biobank research is greater than hypothetical, six indicate the converse relationship, and four are inconclusive. Factual donors, in particular when recruited in health care or otherwise face-to-face with the researcher, are possibly motivated by factors that are less influential in a hypothetical context, such as altruism, trust, and sense of duty. The value of surveys in assessing factual willingness may thus be limited.
Figures
References
-
- Kettis-Lindblad A, Ring L, Viberth E, Hansson MG. Genetic research and donation of tissue samples to biobanks. What do potential sample donors in the Swedish general public think. Eur J Public Health. 2006;16:433–440. - PubMed
-
- Levitt M, Weldon S. A well placed trust?: Public perceptions of the governance of DNA databases. Crit Public Health. 2005;15:311–321.
-
- Caulfield T. Biobanks and Blanket Consent: the proper place of the public good and public perception rationales. King's Law Journal. 2007;18:209–226.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

