Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors
- PMID: 20648635
- PMCID: PMC2925144
- DOI: 10.1002/glia.21015
Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors
Abstract
In utero alcohol exposure can lead to fetal alcohol spectrum disorders, characterized by cognitive and behavioral deficits. In vivo and in vitro studies have shown that ethanol alters neuronal development. We have recently shown that stimulation of M(3) muscarinic receptors in astrocytes increases the synthesis and release of fibronectin, laminin, and plasminogen activator inhibitor-1, causing neurite outgrowth in hippocampal neurons. As M(3) muscarinic receptor signaling in astroglial cells is strongly inhibited by ethanol, we hypothesized that ethanol may also inhibit neuritogenesis in hippocampal neurons induced by carbachol-stimulated astrocytes. In the present study, we report that the effect of carbachol-stimulated astrocytes on hippocampal neuron neurite outgrowth was inhibited in a concentration-dependent manner (25-100 mM) by ethanol. This effect was because of the inhibition of the release of fibronectin, laminin, and plasminogen activator inhibitor-1. Similar effects on neuritogenesis and on the release of astrocyte extracellular proteins were observed after the incubation of astrocytes with carbachol in the presence of 1-butanol, another short-chain alcohol, which like ethanol is a competitive substrate for phospholipase D, but not by tert-butanol, its analog that is not a substrate for this enzyme. This study identifies a potential novel mechanism involved in the developmental effects of ethanol mediated by the interaction of ethanol with cell signaling in astrocytes, leading to an impairment in neuron-astrocyte communication.
(c) 2010 Wiley-Liss, Inc.
Figures




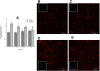


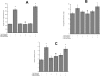


References
-
- Armstrong DM, Bruce G, Hersh LB, Gage FH. Development of cholinergic neurons in the septal/diagonal band complex of the rat. Brain Res. 1987;433(2):249–56. - PubMed
-
- Balduini W, Murphy SD, Costa LG. Developmental changes in muscarinic receptor-stimulated phosphoinositide metabolism in rat brain. J Pharmacol Exp Ther. 1987;241(2):421–7. - PubMed
-
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. - PubMed
-
- Bertrand J, Floyd LL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep. 2005;54(RR-11):1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

