Identification of sites responsible for the potentiating effect of niflumic acid on ClC-Ka kidney chloride channels
- PMID: 20649569
- PMCID: PMC2936838
- DOI: 10.1111/j.1476-5381.2010.00822.x
Identification of sites responsible for the potentiating effect of niflumic acid on ClC-Ka kidney chloride channels
Abstract
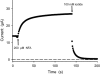
Background and purpose: ClC-K kidney Cl(-) channels are important for renal and inner ear transepithelial Cl(-) transport, and are potentially interesting pharmacological targets. They are modulated by niflumic acid (NFA), a non-steroidal anti-inflammatory drug, in a biphasic way: NFA activates ClC-Ka at low concentrations, but blocks the channel above approximately 1 mM. We attempted to identify the amino acids involved in the activation of ClC-Ka by NFA.
Experimental approach: We used site-directed mutagenesis and two-electrode voltage clamp analysis of wild-type and mutant channels expressed in Xenopus oocytes. Guided by the crystal structure of a bacterial CLC homolog, we screened 97 ClC-Ka mutations for alterations of NFA effects.
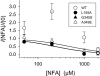
Key results: Mutations of five residues significantly reduced the potentiating effect of NFA. Two of these (G167A and F213A) drastically altered general gating properties and are unlikely to be involved in NFA binding. The three remaining mutants (L155A, G345S and A349E) severely impaired or abolished NFA potentiation.
Conclusions and implications: The three key residues identified (L155, G345, A349) are localized in two different protein regions that, based on the crystal structure of bacterial CLC homologs, are expected to be exposed to the extracellular side of the channel, relatively close to each other, and are thus good candidates for being part of the potentiating NFA binding site. Alternatively, the protein region identified mediates conformational changes following NFA binding. Our results are an important step towards the development of ClC-Ka activators for treating Bartter syndrome types III and IV with residual channel activity.
Figures




References
-
- Adachi S, Uchida S, Ito H, Hata M, Hiroe M, Marumo F, et al. Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle's loop and collecting ducts of rat kidney. J Biol Chem. 1994;269:17677–17683. - PubMed
-
- Akizuki N, Uchida S, Sasaki S, Marumo F. Impaired solute accumulation in inner medulla of Clcnk1–/– mice kidney. Am J Physiol Renal Physiol. 2001;280:F79–F87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

