High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without alpha(2) adrenoceptor activation
- PMID: 20649570
- PMCID: PMC2936839
- DOI: 10.1111/j.1476-5381.2010.00833.x
High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without alpha(2) adrenoceptor activation
Abstract
Background and purpose: Dexmedetomidine, an alpha(2)-adrenoceptor agonist, exhibits anti-nociceptive actions at the spinal cord and enhances the effect of local anaesthetics in the peripheral nervous system. Although the latter action may be attributed in part to inhibition of nerve conduction produced by dexmedetomidine, this has not been fully examined yet.
Experimental approach: We examined the effects of various adrenoceptor agonists including dexmedetomidine, and tetracaine, a local anaesthetic, on compound action potentials (CAPs) recorded from the frog sciatic nerve, using the air-gap method.
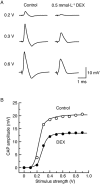
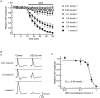
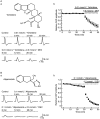
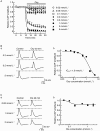
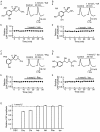
Key results: Dexmedetomidine reversibly and concentration-dependently reduced the peak amplitude of CAPs (IC(50)= 0.40 mmol x L(-1)). This action was not antagonized by two alpha(2)-adrenoceptor antagonists, yohimbine and atipamezole; the latter antagonist itself reduced CAP peak amplitude. Clonidine and oxymetazoline, two other alpha(2)-adrenoceptor agonists, also inhibited CAPs; the maximum effect of clonidine was only 20%, while oxymetazoline was less potent (IC(50)= 1.5 mmol x L(-1)) than dexmedetomidine. On the other hand, (+/-)-adrenaline, (+/-)-noradrenaline, alpha(1)-adrenoceptor agonist (-)-phenylephrine and beta-adrenoceptor agonist (-)-isoprenaline (each 1 mmol x L(-1)) had no effect on CAPs. Tetracaine reversibly reduced CAP peak amplitude (IC(50) of 0.014 mmol x L(-1)).
Conclusions and implications: Dexmedetomidine reduced CAP peak amplitude without alpha(2)-adrenoceptor activation (at concentrations >1000-fold higher than those used as alpha(2) adrenoceptor agonist), with a lower potency than tetracaine. CAPs were inhibited by other alpha(2) adrenoceptor agonists, oxymetazoline and clonidine, and also an alpha(2) adrenoceptor antagonist atipamezole. Thus, some drugs acting on alpha(2) adrenoceptors are able to block nerve conduction.
Figures



Similar articles
-
Conduction block by clonidine is not mediated by alpha2-adrenergic receptors in rat sciatic nerve fibers.Reg Anesth Pain Med. 2000 Nov-Dec;25(6):620-5. doi: 10.1053/rapm.2000.16160. Reg Anesth Pain Med. 2000. PMID: 11097671
-
Compound action potential inhibition produced by various antidepressants in the frog sciatic nerve.Eur J Pharmacol. 2018 Jan 15;819:122-128. doi: 10.1016/j.ejphar.2017.11.047. Epub 2017 Dec 2. Eur J Pharmacol. 2018. PMID: 29203420
-
Dual alpha(2)-adrenergic agonist and alpha(1)-adrenergic antagonist actions of dexmedetomidine on human isolated endothelium-denuded gastroepiploic arteries.Anesth Analg. 2002 Jun;94(6):1434-40, table of contents. doi: 10.1097/00000539-200206000-00010. Anesth Analg. 2002. PMID: 12032002
-
Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor.Anesth Analg. 2008 Jul;107(1):96-101. doi: 10.1213/ane.0b013e318176be73. Anesth Analg. 2008. PMID: 18635472
-
Tramadol, but not its major metabolite (mono-O-demethyl tramadol) depresses compound action potentials in frog sciatic nerves.Br J Pharmacol. 2006 Oct;149(3):319-27. doi: 10.1038/sj.bjp.0706868. Epub 2006 Aug 21. Br J Pharmacol. 2006. PMID: 16921387 Free PMC article.
Cited by
-
Modulating Hyperpolarization-Activated Cation Currents through Small Molecule Perturbations: Magnitude and Gating Control.Biomedicines. 2023 Aug 2;11(8):2177. doi: 10.3390/biomedicines11082177. Biomedicines. 2023. PMID: 37626674 Free PMC article. Review.
-
Multiply repeatable and adjustable on-demand phototriggered local anesthesia.J Control Release. 2017 Apr 10;251:68-74. doi: 10.1016/j.jconrel.2017.01.031. Epub 2017 Jan 31. J Control Release. 2017. PMID: 28153763 Free PMC article.
-
Adding Dexmedetomidine to Articaine Increases the Latency of Thermal Antinociception in Rats.Anesth Prog. 2020 Jun 1;67(2):72-78. doi: 10.2344/anpr-66-04-06. Anesth Prog. 2020. PMID: 32633771 Free PMC article.
-
Additives to local anesthetics for peripheral nerve blockade.Int Anesthesiol Clin. 2011 Fall;49(4):104-16. doi: 10.1097/AIA.0b013e31820e4a49. Int Anesthesiol Clin. 2011. PMID: 21956081 Free PMC article. Review.
-
A comparative evaluation of different doses of dexmedetomidine as an adjuvant to bupivacaine in transversus abdominis plane block for postoperative analgesia in unilateral inguinal hernioplasty.J Anaesthesiol Clin Pharmacol. 2020 Jul-Sep;36(3):398-406. doi: 10.4103/joacp.JOACP_26_19. Epub 2020 Oct 16. J Anaesthesiol Clin Pharmacol. 2020. PMID: 33487910 Free PMC article.
References
-
- Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–268. - PubMed
-
- Bräu ME, Vogel W, Hempelmann G. Fundamental properties of local anesthetics: half-maximal blocking concentrations for tonic block of Na+ and K+ channels in peripheral nerve. Anesth Analg. 1998;87:885–889. - PubMed
-
- Butterworth JF, IV, Strichartz GR. The α2-adrenergic agonists clonidine and guanfacine produce tonic and phasic block of conduction in rat sciatic nerve fibers. Anesth Analg. 1993;76:295–301. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

