2-Phenyl-5-(pyrrolidin-1-yl)-1-(3,4,5-trimethoxybenzyl)-1H-benzimidazole, a benzimidazole derivative, inhibits growth of human prostate cancer cells by affecting tubulin and c-Jun N-terminal kinase
- PMID: 20649571
- PMCID: PMC2936840
- DOI: 10.1111/j.1476-5381.2010.00832.x
2-Phenyl-5-(pyrrolidin-1-yl)-1-(3,4,5-trimethoxybenzyl)-1H-benzimidazole, a benzimidazole derivative, inhibits growth of human prostate cancer cells by affecting tubulin and c-Jun N-terminal kinase
Abstract
Background and purpose: The c-Jun N-terminal kinase (JNK) and tubulin are, frequently, targets for developing anti-cancer drugs. A major obstacle to successful development is P-glycoprotein (P-gp)-mediated resistance. Here, we have assessed a compound that inhibited growth of cancer cells, for effects on JNK and tubulin and as a substrate for P-gp.
Experimental approach: Several pharmacological and biochemical assays were used to characterize signalling pathways of 2-phenyl-5-(pyrrolidin-1-yl)-1-(3,4,5-trimethoxybenzyl)-1H-benzimidazole (PPTMB), a benzimidazole analogue, in prostate cancer cells.
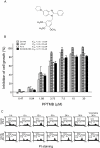
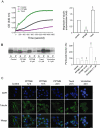
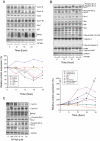
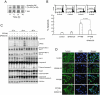
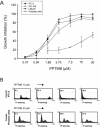
Key results: PPTMB inhibited proliferation of several human prostate cancer cell lines. It displayed similar activity against a P-gp-rich cell line, indicating that PPTMB was not a substrate for P-gp. PPTMB induced G2/M arrest of the cell cycle and subsequent apoptosis, using flow cytometry. Tubulin polymerization assays and Western blot analysis showed that PPTMB directly acted on tubulin and caused disruption of microtubule dynamics, inducing mitotic arrest and sustained high levels of cyclin B1 expression and Cdk1 activation. Subsequently, mitochondria-related apoptotic cascades were induced, including Bcl-2 and Bcl-xL phosphorylation, Mcl-1 down-regulation, truncated Bad formation and activation of caspase-9 and -3. PPTMB stimulated JNK phosphorylation at Thr(183)/Tyr(185). SP600125, a specific JNK inhibitor, significantly inhibited apoptotic signalling, indicating that JNK plays a key role in PPTMB action. PPTMB showed a 10-fold higher potency against prostate cancer cells than normal prostate cells.
Conclusions and implications: PPTMB is an effective anti-cancer agent. It disrupted microtubule dynamics, leading to mitotic arrest of the cell cycle and JNK activation, which in turn stimulated the mitochondria-related apoptotic cascades in prostate cancer cells.
Figures
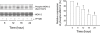
Similar articles
-
Investigation of anti-tumor mechanisms of K2154: characterization of tubulin isotypes, mitotic arrest and apoptotic machinery.Naunyn Schmiedebergs Arch Pharmacol. 2006 Dec;374(3):223-33. doi: 10.1007/s00210-006-0114-x. Epub 2006 Nov 11. Naunyn Schmiedebergs Arch Pharmacol. 2006. PMID: 17102938
-
Repurposing of phentolamine as a potential anticancer agent against human castration-resistant prostate cancer: A central role on microtubule stabilization and mitochondrial apoptosis pathway.Prostate. 2015 Sep;75(13):1454-66. doi: 10.1002/pros.23033. Epub 2015 Jul 14. Prostate. 2015. PMID: 26180030
-
The newly synthesized 2-arylnaphthyridin-4-one, CSC-3436, induces apoptosis of non-small cell lung cancer cells by inhibiting tubulin dynamics and activating CDK1.Cancer Chemother Pharmacol. 2015 Jun;75(6):1303-15. doi: 10.1007/s00280-015-2765-0. Epub 2015 May 7. Cancer Chemother Pharmacol. 2015. PMID: 25947085
-
Benzimidazole hybrids as anticancer drugs: An updated review on anticancer properties, structure-activity relationship, and mechanisms of action (2019-2021).Arch Pharm (Weinheim). 2022 Jun;355(6):e2200051. doi: 10.1002/ardp.202200051. Epub 2022 Apr 6. Arch Pharm (Weinheim). 2022. PMID: 35385159 Review.
-
Recent advances of benzimidazole as anticancer agents.Chem Biol Drug Des. 2023 Aug;102(2):357-376. doi: 10.1111/cbdd.14236. Epub 2023 Apr 3. Chem Biol Drug Des. 2023. PMID: 37009821 Review.
Cited by
-
Targeting of tubulin polymerization and induction of mitotic blockage by Methyl 2-(5-fluoro-2-hydroxyphenyl)-1H-benzo[d]imidazole-5-carboxylate (MBIC) in human cervical cancer HeLa cell.J Exp Clin Cancer Res. 2016 Mar 31;35:58. doi: 10.1186/s13046-016-0332-0. J Exp Clin Cancer Res. 2016. PMID: 27030360 Free PMC article.
-
MAP Kinases and Prostate Cancer.J Signal Transduct. 2012;2012:169170. doi: 10.1155/2012/169170. Epub 2011 Oct 20. J Signal Transduct. 2012. PMID: 22046506 Free PMC article.
-
KUD773, a phenylthiazole derivative, displays anticancer activity in human hormone-refractory prostate cancers through inhibition of tubulin polymerization and anti-Aurora A activity.J Biomed Sci. 2015 Jan 7;22(1):2. doi: 10.1186/s12929-014-0107-x. J Biomed Sci. 2015. PMID: 25563361 Free PMC article.
-
Novel Fluorescent Benzimidazole-Hydrazone-Loaded Micellar Carriers for Controlled Release: Impact on Cell Toxicity, Nuclear and Microtubule Alterations in Breast Cancer Cells.Pharmaceutics. 2023 Jun 16;15(6):1753. doi: 10.3390/pharmaceutics15061753. Pharmaceutics. 2023. PMID: 37376201 Free PMC article.
-
New benzimidazole acridine derivative induces human colon cancer cell apoptosis in vitro via the ROS-JNK signaling pathway.Acta Pharmacol Sin. 2015 Sep;36(9):1074-84. doi: 10.1038/aps.2015.44. Epub 2015 Aug 3. Acta Pharmacol Sin. 2015. PMID: 26235743 Free PMC article.
References
-
- Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. - PubMed
-
- Brozovic A, Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2007;251:1–16. - PubMed
-
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Müller M, Druker BJ, et al. Inhibition of the Abl protein–tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

