A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model
- PMID: 20649573
- PMCID: PMC2936842
- DOI: 10.1111/j.1476-5381.2010.00828.x
A novel, orally active LPA(1) receptor antagonist inhibits lung fibrosis in the mouse bleomycin model
Abstract
Background and purpose: The aim of this study was to assess the potential of an antagonist selective for the lysophosphatidic acid receptor, LPA(1), in treating lung fibrosis We evaluated the in vitro and in vivo pharmacological properties of the high affinity, selective, oral LPA(1)-antagonist (4'-{4-[(R)-1-(2-chloro-phenyl)-ethoxycarbonylamino]-3-methyl-isoxazol-5-yl}-biphenyl-4-yl)-acetic acid (AM966).
Experimental approach: The potency and selectivity of AM966 for LPA(1) receptors was determined in vitro by calcium flux and cell chemotaxis assays using recombinant and native cell cultures. The in vivo efficacy of AM966 to reduce tissue injury, vascular leakage, inflammation and fibrosis was assessed at several time points in the mouse bleomycin model.
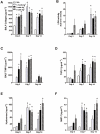
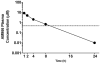
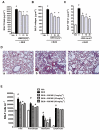
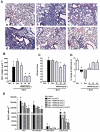
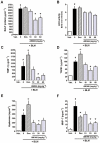
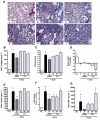
Key results: AM966 was a potent antagonist of LPA(1) receptors, with selectivity for this receptor over the other LPA receptors. In vitro, AM966 inhibited LPA-stimulated intracellular calcium release (IC(50)= 17 nM) from Chinese hamster ovary cells stably expressing human LPA(1) receptors and inhibited LPA-induced chemotaxis (IC(50)= 181 nM) of human IMR-90 lung fibroblasts expressing LPA(1) receptors. AM966 demonstrated a good pharmacokinetic profile following oral dosing in mice. In the mouse, AM966 reduced lung injury, vascular leakage, inflammation and fibrosis at multiple time points following intratracheal bleomycin instillation. AM966 also decreased lactate dehydrogenase activity and tissue inhibitor of metalloproteinase-1, transforming growth factor beta1, hyaluronan and matrix metalloproteinase-7, in bronchoalveolar lavage fluid.
Conclusions and implications: These findings demonstrate that AM966 is a potent, selective, orally bioavailable LPA(1) receptor antagonist that may be beneficial in treating lung injury and fibrosis, as well as other diseases that are characterized by pathological inflammation, oedema and fibrosis.
Figures
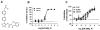
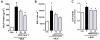

References
-
- Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. - PubMed
-
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

