N-acyl amino acids and N-acyl neurotransmitter conjugates: neuromodulators and probes for new drug targets
- PMID: 20649585
- PMCID: PMC2958632
- DOI: 10.1111/j.1476-5381.2010.00862.x
N-acyl amino acids and N-acyl neurotransmitter conjugates: neuromodulators and probes for new drug targets
Abstract
The myriad functions of lipids as signalling molecules is one of the most interesting fields in contemporary pharmacology, with a host of compounds recognized as mediators of communication within and between cells. The N-acyl conjugates of amino acids and neurotransmitters (NAANs) have recently come to prominence because of their potential roles in the nervous system, vasculature and the immune system. NAAN are compounds such as glycine, GABA or dopamine conjugated with long chain fatty acids. More than 70 endogenous NAAN have been reported although their physiological role remains uncertain, with various NAAN interacting with a low affinity at G protein coupled receptors (GPCR) and ion channels. Regardless of their potential physiological function, NAAN are of great interest to pharmacologists because of their potential as flexible tools to probe new sites on GPCRs, transporters and ion channels. NAANs are amphipathic molecules, with a wide variety of potential fatty acid and headgroup moieties, a combination which provides a rich source of potential ligands engaging novel binding sites and mechanisms for modulation of membrane proteins such as GPCRs, ion channels and transporters. The unique actions of subsets of NAAN on voltage-gated calcium channels and glycine transporters indicate that the wide variety of NAAN may provide a readily exploitable resource for defining new pharmacological targets. Investigation of the physiological roles and pharmacological potential of these simple lipid conjugates is in its infancy, and we believe that there is much to be learnt from their careful study.
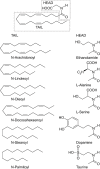
Figures

Similar articles
-
Function and therapeutic potential of N-acyl amino acids.Chem Phys Lipids. 2021 Sep;239:105114. doi: 10.1016/j.chemphyslip.2021.105114. Epub 2021 Jul 2. Chem Phys Lipids. 2021. PMID: 34217720 Review.
-
The pharmacology of mechanogated membrane ion channels.Pharmacol Rev. 1996 Jun;48(2):231-52. Pharmacol Rev. 1996. PMID: 8804105 Review.
-
Phosphorylation of voltage-gated ion channels in rat olfactory receptor neurons.Eur J Neurosci. 2001 Oct;14(7):1056-64. doi: 10.1046/j.0953-816x.2001.01722.x. Eur J Neurosci. 2001. PMID: 11683897
-
Modulation of transmitter release via presynaptic ligand-gated ion channels.Curr Mol Pharmacol. 2008 Jun;1(2):106-29. doi: 10.2174/1874467210801020106. Curr Mol Pharmacol. 2008. PMID: 20021427 Review.
-
Receptors for the excitatory amino acids in the mammalian central nervous system.Prog Neurobiol. 1983;20(3-4):251-71. doi: 10.1016/0301-0082(83)90004-7. Prog Neurobiol. 1983. PMID: 6142499 Review.
Cited by
-
Inhibition of human recombinant T-type calcium channels by N-arachidonoyl 5-HT.Br J Pharmacol. 2012 Nov;167(5):1076-88. doi: 10.1111/j.1476-5381.2012.02047.x. Br J Pharmacol. 2012. PMID: 22624680 Free PMC article.
-
Identification of N-arachidonoyl dopamine as a highly biased ligand at cannabinoid CB1 receptors.Br J Pharmacol. 2016 Jan;173(1):115-27. doi: 10.1111/bph.13341. Epub 2015 Nov 17. Br J Pharmacol. 2016. PMID: 26398720 Free PMC article.
-
Collision-induced dissociation of phenethylamides: role of ion-neutral complexes.Rapid Commun Mass Spectrom. 2017 Sep 15;31(17):1385-1395. doi: 10.1002/rcm.7915. Rapid Commun Mass Spectrom. 2017. PMID: 28558170 Free PMC article.
-
Resveratrol protects from lipopolysaccharide-induced inflammation in the uterus and prevents experimental preterm birth.Mol Hum Reprod. 2017 Aug 1;23(8):571-581. doi: 10.1093/molehr/gax036. Mol Hum Reprod. 2017. PMID: 28810692 Free PMC article.
-
Uncovering metabolic dysregulation in schizophrenia and cannabis use disorder through untargeted plasma lipidomics.Sci Rep. 2024 Dec 28;14(1):31492. doi: 10.1038/s41598-024-83288-5. Sci Rep. 2024. PMID: 39733019 Free PMC article.
References
-
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. - PubMed
-
- Almasi R, Szoke E, Bolcskei K, Varga A, Riedl Z, Sandor Z, et al. Actions of 3-methyl-N-oleoyldopamine, 4-methyl-N-oleoyldopamine and N-oleoylethanolamide on the rat TRPV1 receptor in vitro and in vivo. Life Sci. 2008;82:644–671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

