Biochemical and molecular analysis of carboxylesterase-mediated hydrolysis of cocaine and heroin
- PMID: 20649590
- PMCID: PMC2958638
- DOI: 10.1111/j.1476-5381.2010.00700.x
Biochemical and molecular analysis of carboxylesterase-mediated hydrolysis of cocaine and heroin
Abstract
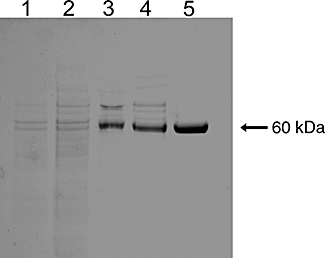
Background and purpose: Carboxylesterases (CEs) metabolize a wide range of xenobiotic substrates including heroin, cocaine, meperidine and the anticancer agent CPT-11. In this study, we have purified to homogeneity human liver and intestinal CEs and compared their ability with hydrolyse heroin, cocaine and CPT-11.
Experimental approach: The hydrolysis of heroin and cocaine by recombinant human CEs was evaluated and the kinetic parameters determined. In addition, microsomal samples prepared from these tissues were subjected to chromatographic separation, and substrate hydrolysis and amounts of different CEs were determined.
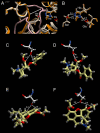
Key results: In contrast to previous reports, cocaine was not hydrolysed by the human liver CE, hCE1 (CES1), either as highly active recombinant protein or as CEs isolated from human liver or intestinal extracts. These results correlated well with computer-assisted molecular modelling studies that suggested that hydrolysis of cocaine by hCE1 (CES1), would be unlikely to occur. However, cocaine, heroin and CPT-11 were all substrates for the intestinal CE, hiCE (CES2), as determined using both the recombinant protein and the tissue fractions. Again, these data were in agreement with the modelling results.
Conclusions and implications: These results indicate that the human liver CE is unlikely to play a role in the metabolism of cocaine and that hydrolysis of this substrate by this class of enzymes is via the human intestinal protein hiCE (CES2). In addition, because no enzyme inhibition is observed at high cocaine concentrations, potentially this route of hydrolysis is important in individuals who overdose on this agent.
Figures
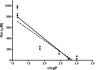
 ), and hCE1 (CES1) (
), and hCE1 (CES1) ( ). The correlation coefficients (r2) for the data sets are indicated on the graph.
). The correlation coefficients (r2) for the data sets are indicated on the graph.
References
-
- Anderson RN. Deaths: leading causes for 1999. Natl Vital Stat Rep. 1999;49:1–88. - PubMed
-
- Bencharit S, Morton CL, Hyatt JL, Kuhn P, Danks MK, Potter PM, et al. Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine. From binding promiscuity to selective inhibition. Chem & Biol. 2003a;10:341–349. - PubMed
-
- Bencharit S, Morton CL, Xue Y, Potter PM, Redinbo MR. Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat Struct Biol. 2003b;10:349–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

