Nucleotide oligomerization domain 1 is a dominant pathway for NOS2 induction in vascular smooth muscle cells: comparison with Toll-like receptor 4 responses in macrophages
- PMID: 20649597
- PMCID: PMC2913099
- DOI: 10.1111/j.1476-5381.2010.00814.x
Nucleotide oligomerization domain 1 is a dominant pathway for NOS2 induction in vascular smooth muscle cells: comparison with Toll-like receptor 4 responses in macrophages
Abstract
Background and purpose: Gram-negative bacteria contain ligands for Toll-like receptor (TLR) 4 and nucleotide oligomerization domain (NOD) 1 receptors. Lipopolysaccharide (LPS) activates TLR4, while peptidoglycan products activate NOD1. Activation of NOD1 by the specific agonist FK565 results in a profound vascular dysfunction and experimental shock in vivo.
Experimental approach: Here, we have analysed a number of pharmacological inhibitors to characterize the role of key signalling pathways in the induction of NOS2 following TLR4 or NOD1 activation.
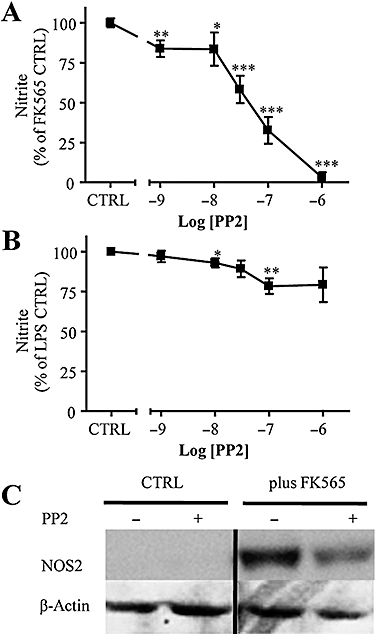
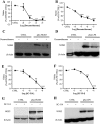
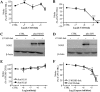
Key results: Vascular smooth muscle (VSM) cells expressed NOD1 mRNA and protein, and, after challenge with Escherichia coli or FK565, NOS2 protein and activity were induced. Macrophages had negligible levels of NOD1 and were unaffected by FK565, but responded to E. coli and LPS by releasing increased NO and expression of NOS2 protein. Classic pharmacological inhibitors for NF-kappaB (SC-514) and mitogen-activated protein kinase (SB203580, PD98059) signalling pathways inhibited responses in both cell types regardless of agonist. While TLR4-mediated responses in macrophages were specifically inhibited by the pan-caspase inhibitor z-VAD-fmk and the PKC inhibitor Gö6976, NOD1-mediated responses in VSM cells were inhibited by the Rip2 inhibitor PP2.
Conclusions and implications: Our findings suggest a selective role for NOD1 in VSM cells, and highlight NOD1 as a potential novel therapeutic target for the treatment of vascular inflammation.
Figures
References
-
- Argast GM, Fausto N, Campbell JS. Inhibition of RIP2/RIck/CARDIAK activity by pyridinyl imidazole inhibitors of p38 MAPK. Mol Cell Biochem. 2005;268:129–140. - PubMed
-
- Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal. 2005;17:385–394. - PubMed
-
- Borsello T, Bonny C. Use of cell-permeable peptides to prevent neuronal degeneration. Trends Mol Med. 2004;10:239–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

