Resistance to endotoxic shock in mice lacking natriuretic peptide receptor-A
- PMID: 20649600
- PMCID: PMC2913103
- DOI: 10.1111/j.1476-5381.2010.00830.x
Resistance to endotoxic shock in mice lacking natriuretic peptide receptor-A
Abstract
Background and purpose: Excessive production of nitric oxide (NO) by inducible NO synthase (iNOS) is thought to underlie the vascular dysfunction, systemic hypotension and organ failure that characterize endotoxic shock. Plasma levels of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP) are raised in animal models and humans with endotoxic shock and correlate with the associated cardiovascular dysfunction. Since both NO and natriuretic peptides play important roles in cardiovascular homeostasis via activation of guanylate cyclase-linked receptors, we used mice lacking natriuretic peptide receptor (NPR)-A (NPR1) to establish if natriuretic peptides contribute to the cardiovascular dysfunction present in endotoxic shock.
Experimental approach: Wild-type (WT) and NPR-A knockout (KO) mice were exposed to lipopolysaccharide (LPS) and vascular dysfunction (in vitro and in vivo), production of pro-inflammatory cytokines, and iNOS expression and activity were evaluated.
Key results: LPS-treated WT animals exhibited a marked fall in mean arterial blood pressure (MABP) whereas NPR-A KO mice maintained MABP throughout. LPS administration caused a greater suppression of vascular responses to the thromboxane-mimetic U46619, ANP, acetylcholine and the NO-donor spermine-NONOate in WT versus NPR-A KO mice. This differential effect on vascular function was paralleled by reduced pro-inflammatory cytokine production, iNOS expression and activity (plasma [NO(x)] and cyclic GMP).
Conclusions and implications: These observations suggest that NPR-A activation by natriuretic peptides facilitates iNOS expression and contributes to the vascular dysfunction characteristic of endotoxic shock. Pharmacological interventions that target the natriuretic peptide system may represent a novel approach to treat this life-threatening condition.
Figures
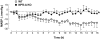
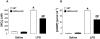


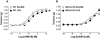
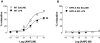
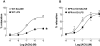
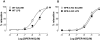
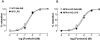
Similar articles
-
Vascular natriuretic peptide receptor-linked particulate guanylate cyclases are modulated by nitric oxide-cyclic GMP signalling.Br J Pharmacol. 2003 Aug;139(7):1289-96. doi: 10.1038/sj.bjp.0705365. Br J Pharmacol. 2003. PMID: 12890708 Free PMC article.
-
Organ-specific changes in vascular reactivity and roles of inducible nitric oxide synthase and endothelin-1 in a rabbit endotoxic shock model.J Trauma Acute Care Surg. 2018 Oct;85(4):725-733. doi: 10.1097/TA.0000000000002036. J Trauma Acute Care Surg. 2018. PMID: 30086070
-
Contribution of iNOS/sGC/PKG pathway, COX-2, CYP4A1, and gp91(phox) to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against vasodilation, hypotension, tachycardia, and inflammation in a rat model of septic shock.Nitric Oxide. 2013 Sep 1;33:18-41. doi: 10.1016/j.niox.2013.05.001. Epub 2013 May 14. Nitric Oxide. 2013. PMID: 23684565 Free PMC article.
-
[Molecular biology and pharmacology of natriuretic peptide system].Nihon Rinsho. 1993 Jun;51(6):1548-56. Nihon Rinsho. 1993. PMID: 8100590 Review. Japanese.
-
Natriuretic peptide receptor-C signaling and regulation.Peptides. 2005 Jun;26(6):1044-59. doi: 10.1016/j.peptides.2004.09.023. Epub 2005 Apr 8. Peptides. 2005. PMID: 15911072 Review.
Cited by
-
A novel synthetic mono-carbonyl analogue of curcumin, A13, exhibits anti-inflammatory effects in vivo by inhibition of inflammatory mediators.Inflammation. 2012 Apr;35(2):594-604. doi: 10.1007/s10753-011-9350-4. Inflammation. 2012. PMID: 21614553
-
A novel compound C12 inhibits inflammatory cytokine production and protects from inflammatory injury in vivo.PLoS One. 2011;6(9):e24377. doi: 10.1371/journal.pone.0024377. Epub 2011 Sep 8. PLoS One. 2011. PMID: 21931698 Free PMC article.
-
C-Type Natriuretic Peptide Induces Anti-contractile Effect Dependent on Nitric Oxide, Oxidative Stress, and NPR-B Activation in Sepsis.Front Physiol. 2016 Jun 23;7:226. doi: 10.3389/fphys.2016.00226. eCollection 2016. Front Physiol. 2016. PMID: 27445832 Free PMC article.
-
Nitric oxide and redox mechanisms in the immune response.J Leukoc Biol. 2011 Jun;89(6):873-91. doi: 10.1189/jlb.1010550. Epub 2011 Jan 13. J Leukoc Biol. 2011. PMID: 21233414 Free PMC article. Review.
-
B-type natriuretic peptide is upregulated by c-Jun N-terminal kinase and contributes to septic hypotension.JCI Insight. 2020 Apr 23;5(8):e133675. doi: 10.1172/jci.insight.133675. JCI Insight. 2020. PMID: 32324169 Free PMC article.
References
-
- Ahluwalia A, MacAllister RJ, Hobbs AJ. Vascular actions of natriuretic peptides. Cyclic GMP-dependent and -independent mechanisms. Basic Res Cardiol. 2004;99:83–89. - PubMed
-
- Aiura K, Ueda M, Endo M, Kitajima M. Circulating concentrations and physiologic role of atrial natriuretic peptide during endotoxic shock in the rat. Crit Care Med. 1995;23:1898–1906. - PubMed
-
- Annane D, Sanquer S, Sebille V, Faye A, Djuranovic D, Raphael JC, et al. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet. 2000;355:1143–1148. - PubMed
-
- Chauhan SD, Seggara G, Vo PA, MacAllister RJ, Hobbs AJ, Ahluwalia A. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB J. 2003;17:773–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

