Degradation of HER2/neu by ANT2 shRNA suppresses migration and invasiveness of breast cancer cells
- PMID: 20650008
- PMCID: PMC2919502
- DOI: 10.1186/1471-2407-10-391
Degradation of HER2/neu by ANT2 shRNA suppresses migration and invasiveness of breast cancer cells
Abstract
Background: In breast cancer, the HER2/neu oncoprotein, which belongs to the epidermal growth factor receptor family, may trigger activation of the phosphoinositide-3 kinase (PI3K)/Akt pathway, which controls cell proliferation, survival, migration, and invasion. In this study, we examined the question of whether or not adenine nucleotide translocase 2 (ANT2) short hairpin RNA (shRNA)-mediated down-regulation of HER2/neu and inhibitory effects on the PI3K/Akt signaling pathway suppressed migration and invasiveness of breast cancer cells.
Methods: We utilized an ANT2 vector-based RNA interference approach to inhibition of ANT2 expression, and the HER2/neu-overexpressing human breast cancer cell line, SK-BR3, was used throughout the study.
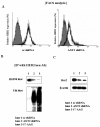
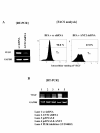
Results: In this study, ANT2 shRNA decreased HER2/neu protein levels by promoting degradation of HER2/neu protein through dissociation from heat shock protein 90 (HSP90). As a result, ANT2 shRNA induced inhibitory effects on the PI3K/Akt signaling pathway. Inhibition of PI3K/Akt signaling by ANT2 shRNA caused down-regulation of membrane-type 1 matrix metalloproteinase (MT1-MMP) and vascular endothelial growth factor (VEGF) expression, decreased matrix metalloproteinase 2 (MMP2) and MMP9 activity, and suppressed migration and invasion of breast cancer cells.
Conclusions: These results indicate that knock-down of ANT2 by shRNA down-regulates HER2/neu through suppression of HSP90's function and inhibits the PI3K/Akt signaling pathway, resulting ultimately in suppressed migration and invasion of breast cancer cells.
Figures




Similar articles
-
ANT2 shRNA downregulates miR-19a and miR-96 through the PI3K/Akt pathway and suppresses tumor growth in hepatocellular carcinoma cells.Exp Mol Med. 2016 Mar 25;48(3):e222. doi: 10.1038/emm.2015.126. Exp Mol Med. 2016. PMID: 27012708 Free PMC article.
-
Short-hairpin RNA-induced suppression of adenine nucleotide translocase-2 in breast cancer cells restores their susceptibility to TRAIL-induced apoptosis by activating JNK and modulating TRAIL receptor expression.Mol Cancer. 2010 Sep 28;9:262. doi: 10.1186/1476-4598-9-262. Mol Cancer. 2010. PMID: 20875141 Free PMC article.
-
Targeting Adenine Nucleotide Translocase-2 (ANT2) to Overcome Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in Non-Small Cell Lung Cancer.Mol Cancer Ther. 2016 Jun;15(6):1387-96. doi: 10.1158/1535-7163.MCT-15-0089. Epub 2016 Feb 16. Mol Cancer Ther. 2016. PMID: 26883272
-
The achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention.Cell Cycle. 2004 Jan;3(1):51-60. Cell Cycle. 2004. PMID: 14657666 Review.
-
The role of HER2 in early breast cancer metastasis and the origins of resistance to HER2-targeted therapies.Exp Mol Pathol. 2009 Aug;87(1):1-11. doi: 10.1016/j.yexmp.2009.05.001. Epub 2009 May 18. Exp Mol Pathol. 2009. PMID: 19450579 Free PMC article. Review.
Cited by
-
HER2 mRNA status contributes to the discrepancy between gene amplification and protein overexpression in gastric cancer.Dig Dis Sci. 2014 Feb;59(2):328-35. doi: 10.1007/s10620-013-2925-1. Epub 2013 Nov 2. Dig Dis Sci. 2014. PMID: 24185685
-
In vivo assessment of simultaneous G1 cyclins silencing by a tumor-specific bidirectional promoter on the mammary tumor in nude mice.Front Vet Sci. 2022 Aug 22;9:914311. doi: 10.3389/fvets.2022.914311. eCollection 2022. Front Vet Sci. 2022. PMID: 36072388 Free PMC article.
-
BMP9 regulates cross-talk between breast cancer cells and bone marrow-derived mesenchymal stem cells.Cell Oncol (Dordr). 2014 Oct;37(5):363-75. doi: 10.1007/s13402-014-0197-1. Epub 2014 Sep 11. Cell Oncol (Dordr). 2014. PMID: 25209393
-
ANT2 shRNA downregulates miR-19a and miR-96 through the PI3K/Akt pathway and suppresses tumor growth in hepatocellular carcinoma cells.Exp Mol Med. 2016 Mar 25;48(3):e222. doi: 10.1038/emm.2015.126. Exp Mol Med. 2016. PMID: 27012708 Free PMC article.
-
Recent Updates on the Efficacy of Mitocans in Photo/Radio-therapy for Targeting Metabolism in Chemo/Radio-resistant Cancers: Nanotherapeutics.Curr Med Chem. 2025;32(11):2156-2182. doi: 10.2174/0109298673259347231019121757. Curr Med Chem. 2025. PMID: 38018190 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

