Spontaneous rhythmogenic capabilities of sympathetic neuronal assemblies in the rat spinal cord slice
- PMID: 20650307
- PMCID: PMC2989444
- DOI: 10.1016/j.neuroscience.2010.07.007
Spontaneous rhythmogenic capabilities of sympathetic neuronal assemblies in the rat spinal cord slice
Abstract
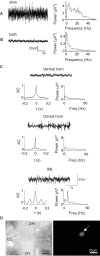
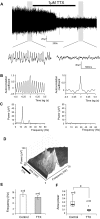
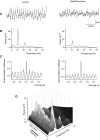
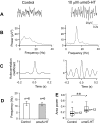
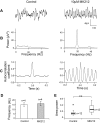
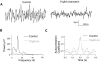
Neuronal networks generating rhythmic activity as an emergent property are common throughout the nervous system. Some are responsible for rhythmic behaviours, as is the case for the spinal cord locomotor networks; however, for others the function is more subtle and usually involves information processing and/or transfer. An example of the latter is sympathetic nerve activity, which is synchronized into rhythmic bursts in vivo. This arrangement is postulated to offer improved control of target organ responses compared to tonic nerve activity. Traditionally, oscillogenic circuits in the brainstem are credited with generating these rhythms, despite evidence for the persistence of some frequencies in spinalized preparations. Here, we show that rhythmic population activity can be recorded from the intermediolateral cell column (IML) of thoracic spinal cord slices. Recorded in slices from 10- to 12-day-old rats, this activity was manifest as 8-22 Hz oscillations in the field potential and was spatially restricted to the IML. Oscillations often occurred spontaneously, but could also be induced by application of 5-HT, α-methyl 5-HT or MK212. These agents also significantly increased the strength of spontaneous oscillations. Rhythmic activity was abolished by TTX and attenuated by application of gap junction blockers or by antagonists of GABA(A) receptors. Together these data indicate that this rhythm is an emergent feature of a population of spinal neurons coupled by gap junctions. This work questions the assumption that sympathetic rhythms are dependent on supraspinal pacemaker circuits, by highlighting a surprisingly strong rhythmogenic capability of the reduced sympathetic networks of the spinal cord slice.
Copyright © 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
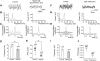
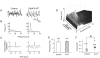
Similar articles
-
Membrane properties and synaptic potentials in rat sympathetic preganglionic neurons studied in horizontal spinal cord slices in vitro.J Auton Nerv Syst. 1995 May 17;53(1):1-15. doi: 10.1016/0165-1838(94)00161-c. J Auton Nerv Syst. 1995. PMID: 7673598
-
Electrotonic coupling between rat sympathetic preganglionic neurones in vitro.J Physiol. 1996 Sep 1;495 ( Pt 2)(Pt 2):491-502. doi: 10.1113/jphysiol.1996.sp021609. J Physiol. 1996. PMID: 8887759 Free PMC article.
-
Noradrenaline induces rhythmic bursting in sympathetic preganglionic neurons.Brain Res. 1987 Sep 8;420(1):147-51. doi: 10.1016/0006-8993(87)90250-2. Brain Res. 1987. PMID: 3676749
-
Rhythmic motor activity in thin transverse slice preparations of the fetal rat spinal cord.J Neurophysiol. 2004 Jul;92(1):648-52. doi: 10.1152/jn.01029.2003. Epub 2004 Mar 17. J Neurophysiol. 2004. PMID: 15028747
-
How sympathetic are your spinal cord circuits?Exp Physiol. 2015 Apr 1;100(4):365-71. doi: 10.1113/EP085031. Exp Physiol. 2015. PMID: 25655449 Review.
Cited by
-
Neurochemical and electrical modulation of the locus coeruleus: contribution to CO2drive to breathe.Front Physiol. 2014 Aug 5;5:288. doi: 10.3389/fphys.2014.00288. eCollection 2014. Front Physiol. 2014. PMID: 25183958 Free PMC article. Review.
-
Physiologic regulation of heart rate and blood pressure involves connexin 36-containing gap junctions.FASEB J. 2017 Sep;31(9):3966-3977. doi: 10.1096/fj.201600919RR. Epub 2017 May 22. FASEB J. 2017. PMID: 28533325 Free PMC article.
-
Spinal Shox2 interneuron interconnectivity related to function and development.Elife. 2018 Dec 31;7:e42519. doi: 10.7554/eLife.42519. Elife. 2018. PMID: 30596374 Free PMC article.
-
Nitric Oxide Orchestrates a Power-Law Modulation of Sympathetic Firing Behaviors in Neonatal Rat Spinal Cords.Front Physiol. 2018 Mar 6;9:163. doi: 10.3389/fphys.2018.00163. eCollection 2018. Front Physiol. 2018. PMID: 29559921 Free PMC article.
-
Cholinergic-mediated coordination of rhythmic sympathetic and motor activities in the newborn rat spinal cord.PLoS Biol. 2018 Jul 9;16(7):e2005460. doi: 10.1371/journal.pbio.2005460. eCollection 2018 Jul. PLoS Biol. 2018. PMID: 29985914 Free PMC article.
References
-
- Allen A.M., Adams J.M., Guyenet P.G. Role of the spinal cord in generating the 2- to 6-Hz rhythm in rat sympathetic outflow. Am J Physiol. 1993;264:R938–R945. - PubMed
-
- Barman S.M., Gebber G.L. “Rapid” rhythmic discharges of sympathetic nerves: sources, mechanisms of generation, and physiological relevance. J Biol Rhythms. 2000;15(5):365–379. - PubMed
-
- Cobb S.R., Buhl E.H., Halasy K., Paulsen O., Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

