Neonatal and fetal exposure to trans-fatty acids retards early growth and adiposity while adversely affecting glucose in mice
- PMID: 20650350
- PMCID: PMC2910901
- DOI: 10.1016/j.nutres.2010.06.006
Neonatal and fetal exposure to trans-fatty acids retards early growth and adiposity while adversely affecting glucose in mice
Abstract
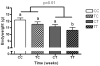
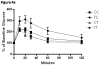
Industrially produced trans-fatty acids (TFAs) consumed in Western diets are incorporated into maternal and fetal tissues and are passed linearly to offspring via breast milk. We hypothesized that TFA exposure in utero and during lactation in infants would promote obesity and poor glycemic control as compared with unmodified fatty acids. We further hypothesized that in utero exposure alone may program for these outcomes in adulthood. To test this hypothesis, we fed female C57/BL6 mice identical Western diets that differed only in cis- or trans-isomers of C18:1 and then aimed to determine whether maternal transfer of TFAs through pregnancy and lactation alters growth, body composition, and glucose metabolism. Mice were unexposed, exposed during pregnancy, during lactation, or throughout pregnancy and lactation to TFA. Body weight and composition (by computed tomography) and glucose metabolism were assessed at weaning and adulthood. Trans-fatty acid exposure through breast milk caused significant early growth retardation (P < .001) and higher fasting glucose (P = .01), but insulin sensitivity was not different. Elevated plasma insulin-like growth factor-1 in mice consuming TFA-enriched milk (P = .02) may contribute to later catch-up growth and leanness and preserved peripheral insulin sensitivity observed in these mice. Mice exposed to TFA in utero underwent rapid early neonatal growth with TFA-free breast milk and had significantly impaired insulin sensitivity (P < .05) and greater abdominal fat (P = .01). We conclude that very early catch-up growth resulted in impaired peripheral insulin sensitivity in this model of diet-related fetal and neonatal programming. Trans-fatty acid surprisingly retarded growth and adiposity while still adversely affecting glucose metabolism.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

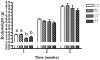

Similar articles
-
Maternal intake of trans-unsaturated or interesterified fatty acids during pregnancy and lactation modifies mitochondrial bioenergetics in the liver of adult offspring in mice.Br J Nutr. 2017 Jul;118(1):41-52. doi: 10.1017/S0007114517001817. Epub 2017 Aug 11. Br J Nutr. 2017. PMID: 28797310
-
Effects of a normolipidic diet containing trans fatty acids during perinatal period on the growth, hippocampus fatty acid profile, and memory of young rats according to sex.Nutrition. 2012 Apr;28(4):458-64. doi: 10.1016/j.nut.2011.08.007. Epub 2011 Dec 14. Nutrition. 2012. PMID: 22169117
-
Trans fatty acids in maternal milk lead to cardiac insulin resistance in adult offspring.Nutrition. 2008 Jul-Aug;24(7-8):727-32. doi: 10.1016/j.nut.2008.03.006. Epub 2008 May 21. Nutrition. 2008. PMID: 18499400
-
Fetal and neonatal exposure to trans-fatty acids impacts on susceptibility to atherosclerosis in apo E*3 Leiden mice.Br J Nutr. 2017 Feb;117(3):377-385. doi: 10.1017/S0007114517000137. Epub 2017 Feb 22. Br J Nutr. 2017. PMID: 28222826
-
Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring.J Nutr Biochem. 2015 Feb;26(2):99-111. doi: 10.1016/j.jnutbio.2014.10.001. Epub 2014 Oct 12. J Nutr Biochem. 2015. PMID: 25459884 Review.
Cited by
-
Maternal fat intake in rats alters 20:4n-6 and 22:6n-3 status and the epigenetic regulation of Fads2 in offspring liver.J Nutr Biochem. 2013 Jul;24(7):1213-20. doi: 10.1016/j.jnutbio.2012.09.005. Epub 2012 Oct 27. J Nutr Biochem. 2013. PMID: 23107313 Free PMC article.
-
Early life factors and type 2 diabetes mellitus.J Diabetes Res. 2013;2013:485082. doi: 10.1155/2013/485082. Epub 2013 Dec 16. J Diabetes Res. 2013. PMID: 24455747 Free PMC article. Review.
-
Lasting effects on body weight and mammary gland gene expression in female mice upon early life exposure to n-3 but not n-6 high-fat diets.PLoS One. 2013;8(2):e55603. doi: 10.1371/journal.pone.0055603. Epub 2013 Feb 7. PLoS One. 2013. PMID: 23409006 Free PMC article.
-
Evidence for transgenerational metabolic programming in Drosophila.Dis Model Mech. 2013 Sep;6(5):1123-32. doi: 10.1242/dmm.011924. Epub 2013 May 2. Dis Model Mech. 2013. PMID: 23649823 Free PMC article.
-
Effects of a High Trans Fatty Acid Diet on Kidney-, Liver-, and Heart-Associated Diseases in a Rabbit Model.Metabolites. 2024 Aug 8;14(8):442. doi: 10.3390/metabo14080442. Metabolites. 2024. PMID: 39195538 Free PMC article.
References
-
- Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. - PubMed
-
- Singhal A, Lucas A. Early origins of cardiovascular disease: Is there a unifying hypothesis? Lancet. 2004;363:1642–1645. - PubMed
-
- Dunger DB, Salgin B, Ong KK. Session 7: Early nutrition and later health early developmental pathways of obesity and diabetes risk. Proc Nutr Soc. 2007;66:451–457. - PubMed
-
- Salmeron J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73:1019–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

