Ion channels and signaling in the pituitary gland
- PMID: 20650859
- PMCID: PMC3365841
- DOI: 10.1210/er.2010-0005
Ion channels and signaling in the pituitary gland
Abstract
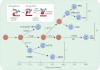
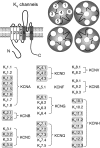
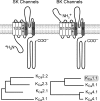
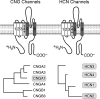
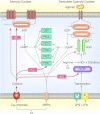
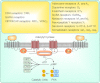
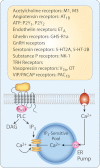
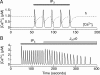
Endocrine pituitary cells are neuronlike; they express numerous voltage-gated sodium, calcium, potassium, and chloride channels and fire action potentials spontaneously, accompanied by a rise in intracellular calcium. In some cells, spontaneous electrical activity is sufficient to drive the intracellular calcium concentration above the threshold for stimulus-secretion and stimulus-transcription coupling. In others, the function of these action potentials is to maintain the cells in a responsive state with cytosolic calcium near, but below, the threshold level. Some pituitary cells also express gap junction channels, which could be used for intercellular Ca(2+) signaling in these cells. Endocrine cells also express extracellular ligand-gated ion channels, and their activation by hypothalamic and intrapituitary hormones leads to amplification of the pacemaking activity and facilitation of calcium influx and hormone release. These cells also express numerous G protein-coupled receptors, which can stimulate or silence electrical activity and action potential-dependent calcium influx and hormone release. Other members of this receptor family can activate calcium channels in the endoplasmic reticulum, leading to a cell type-specific modulation of electrical activity. This review summarizes recent findings in this field and our current understanding of the complex relationship between voltage-gated ion channels, ligand-gated ion channels, gap junction channels, and G protein-coupled receptors in pituitary cells.
Figures







References
-
- Kidokoro Y 1975 Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature 258:741–742 - PubMed
-
- Stojilkovic SS 2008 Ion channels, transporters, and electrical signaling. In: Coon PM, ed. Neuroscience in medicine. Totowa, NJ: Humana Press
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

