Loss of beta1-integrin enhances TGF-beta1-induced collagen expression in epithelial cells via increased alphavbeta3-integrin and Rac1 activity
- PMID: 20650890
- PMCID: PMC2945568
- DOI: 10.1074/jbc.M110.105700
Loss of beta1-integrin enhances TGF-beta1-induced collagen expression in epithelial cells via increased alphavbeta3-integrin and Rac1 activity
Abstract
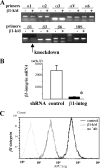
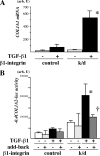
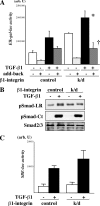
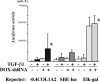
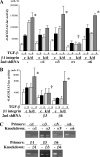
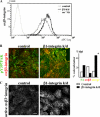
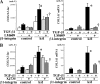
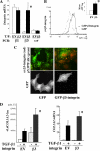
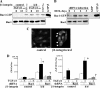
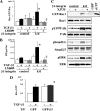
Transforming growth factor β (TGF-β) promotes tissue fibrosis via the receptor-specific Smad pathway and non-canonical pathways. We recently reported that TGF-β1-stimulated collagen expression by cultured kidney cells requires integrin-dependent activation of focal adhesion kinase (FAK) and consequent ERK MAP kinase activity leading to Smad3 linker region phosphorylation. Here, we defined a role for αvβ3-integrin in this non-canonical pathway. A human kidney tubular cell line in which β1-integrin was knocked down (β1-k/d) demonstrated enhanced type I collagen mRNA expression and promoter activity. A second shRNA to either αv-integrin or β3-integrin, but not to another αv-binding partner, β6-integrin, abrogated the enhanced COL1A2 promoter activity in β1-k/d cells. Although αvβ3-integrin surface expression levels were not different, αvβ3-integrins colocalized with sites of focal adhesion significantly more in β1-k/d cells, and activated αvβ3-integrin was detected only in β1-k/d cells. Further, the collagen response was decreased by a function-blocking antibody or a peptide inhibitor of αvβ3-integrin. In cells lacking αvβ3-integrin, the responses were attenuated, whereas the response was enhanced in αvβ3-overexpressing cells. Rac1 and ERK, previously defined mediators for this non-canonical pathway, showed increased activities in β1-k/d cells. Finally, inhibition of αvβ3-integrin decreased Rac1 activity and COL1A2 promoter activity in β1-k/d cells. Together, our results indicate that decreasing β1 chain causes αvβ3-integrin to become functionally dominant and promotes renal cell fibrogenesis via Rac1-mediated ERK activity.
Figures
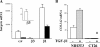
Similar articles
-
MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion-dependent phosphorylation of FAK tyrosine 397.J Cell Sci. 2007 Dec 1;120(Pt 23):4230-40. doi: 10.1242/jcs.03492. J Cell Sci. 2007. PMID: 18032789
-
Transforming growth factor beta1 induces alphavbeta3 integrin expression in human lung fibroblasts via a beta3 integrin-, c-Src-, and p38 MAPK-dependent pathway.J Biol Chem. 2008 May 9;283(19):12898-908. doi: 10.1074/jbc.M708226200. Epub 2008 Mar 18. J Biol Chem. 2008. PMID: 18353785
-
Microvessel vascular smooth muscle cells contribute to collagen type I deposition through ERK1/2 MAP kinase, alphavbeta3-integrin, and TGF-beta1 in response to ANG II and high glucose.Am J Physiol Heart Circ Physiol. 2008 Jul;295(1):H69-76. doi: 10.1152/ajpheart.00341.2008. Epub 2008 May 2. Am J Physiol Heart Circ Physiol. 2008. PMID: 18456735 Free PMC article.
-
Giving off mixed signals--distinct functions of alpha5beta1 and alphavbeta3 integrins in regulating cell behaviour.IUBMB Life. 2009 Jul;61(7):731-8. doi: 10.1002/iub.200. IUBMB Life. 2009. PMID: 19514020 Free PMC article. Review.
-
Integrin β1: A Mechanosignaling Sensor Essential for Connective Tissue Deposition by Fibroblasts.Adv Wound Care (New Rochelle). 2013 May;2(4):160-166. doi: 10.1089/wound.2012.0365. Adv Wound Care (New Rochelle). 2013. PMID: 24527339 Free PMC article. Review.
Cited by
-
Expression and activity of Rac1 is negatively affected in the dehydroepiandrosterone induced polycystic ovary of mouse.J Ovarian Res. 2014 Mar 14;7:32. doi: 10.1186/1757-2215-7-32. J Ovarian Res. 2014. PMID: 24628852 Free PMC article.
-
Fibrillar Type I Collagen Enhances the Differentiation and Proliferation of Myofibroblasts by Lowering α2β1 Integrin Expression in Cardiac Fibrosis.Biomed Res Int. 2017;2017:1790808. doi: 10.1155/2017/1790808. Epub 2017 Jan 30. Biomed Res Int. 2017. PMID: 28251149 Free PMC article.
-
Lung-specific loss of the laminin α3 subunit confers resistance to mechanical injury.J Cell Sci. 2011 Sep 1;124(Pt 17):2927-37. doi: 10.1242/jcs.080911. J Cell Sci. 2011. PMID: 21878500 Free PMC article.
-
Podocyte injury-driven intracapillary plasminogen activator inhibitor type 1 accelerates podocyte loss via uPAR-mediated β1-integrin endocytosis.Am J Physiol Renal Physiol. 2015 Mar 15;308(6):F614-26. doi: 10.1152/ajprenal.00616.2014. Epub 2015 Jan 13. Am J Physiol Renal Physiol. 2015. PMID: 25587125 Free PMC article.
-
Integrin Crosstalk Contributes to the Complexity of Signalling and Unpredictable Cancer Cell Fates.Cancers (Basel). 2020 Jul 15;12(7):1910. doi: 10.3390/cancers12071910. Cancers (Basel). 2020. PMID: 32679769 Free PMC article. Review.
References
-
- Blobe G. C., Schiemann W. P., Lodish H. F. (2000) N. Engl. J. Med. 342, 1350–1358 - PubMed
-
- Schmierer B., Hill C. S. (2007) Nat. Rev. Mol. Cell Biol. 8, 970–982 - PubMed
-
- Moustakas A., Heldin C. H. (2005) J. Cell Sci. 118, 3573–3584 - PubMed
-
- Schnaper H. W., Hayashida T., Hubchak S. C., Poncelet A. C. (2003) Am. J. Physiol. Renal Physiol. 284, F243–F252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

