Both forward and reverse TCA cycles operate in green sulfur bacteria
- PMID: 20650900
- PMCID: PMC2975208
- DOI: 10.1074/jbc.M110.157834
Both forward and reverse TCA cycles operate in green sulfur bacteria
Abstract
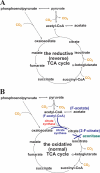
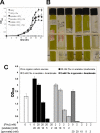
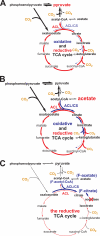
The anoxygenic green sulfur bacteria (GSBs) assimilate CO(2) autotrophically through the reductive (reverse) tricarboxylic acid (RTCA) cycle. Some organic carbon sources, such as acetate and pyruvate, can be assimilated during the phototrophic growth of the GSBs, in the presence of CO(2) or HCO(3)(-). It has not been established why the inorganic carbonis required for incorporating organic carbon for growth and how the organic carbons are assimilated. In this report, we probed carbon flux during autotrophic and mixotrophic growth of the GSB Chlorobaculum tepidum. Our data indicate the following: (a) the RTCA cycle is active during autotrophic and mixotrophic growth; (b) the flux from pyruvate to acetyl-CoA is very low and acetyl-CoA is synthesized through the RTCA cycle and acetate assimilation; (c) pyruvate is largely assimilated through the RTCA cycle; and (d) acetate can be assimilated via both of the RTCA as well as the oxidative (forward) TCA (OTCA) cycle. The OTCA cycle revealed herein may explain better cell growth during mixotrophic growth with acetate, as energy is generated through the OTCA cycle. Furthermore, the genes specific for the OTCA cycle are either absent or down-regulated during phototrophic growth, implying that the OTCA cycle is not complete, and CO(2) is required for the RTCA cycle to produce metabolites in the TCA cycle. Moreover, CO(2) is essential for assimilating acetate and pyruvate through the CO(2)-anaplerotic pathway and pyruvate synthesis from acetyl-CoA.
Figures

Similar articles
-
Metabolic flux analysis of the mixotrophic metabolisms in the green sulfur bacterium Chlorobaculum tepidum.J Biol Chem. 2010 Dec 10;285(50):39544-50. doi: 10.1074/jbc.M110.162958. Epub 2010 Oct 11. J Biol Chem. 2010. PMID: 20937805 Free PMC article.
-
Deletion of Re-citrate synthase allows for analysis of contributions of tricarboxylic acid cycle directionality to the growth of Heliomicrobium modesticaldum.Appl Environ Microbiol. 2025 Apr 23;91(4):e0177224. doi: 10.1128/aem.01772-24. Epub 2025 Mar 6. Appl Environ Microbiol. 2025. PMID: 40047422 Free PMC article.
-
High CO2 levels drive the TCA cycle backwards towards autotrophy.Nature. 2021 Apr;592(7856):784-788. doi: 10.1038/s41586-021-03456-9. Epub 2021 Apr 21. Nature. 2021. PMID: 33883741
-
A reverse KREBS cycle in photosynthesis: consensus at last.Photosynth Res. 1990;24:47-53. Photosynth Res. 1990. PMID: 11540925 Review.
-
Unfamiliar metabolic links in the central carbon metabolism.J Biotechnol. 2014 Dec 20;192 Pt B:314-22. doi: 10.1016/j.jbiotec.2014.02.015. Epub 2014 Feb 24. J Biotechnol. 2014. PMID: 24576434 Review.
Cited by
-
Gene expression system in green sulfur bacteria by conjugative plasmid transfer.PLoS One. 2013 Nov 27;8(11):e82345. doi: 10.1371/journal.pone.0082345. eCollection 2013. PLoS One. 2013. PMID: 24312414 Free PMC article.
-
A Novel Microbialite-Associated Phototrophic Chloroflexi Lineage Exhibiting a Quasi-Clonal Pattern along Depth.Genome Biol Evol. 2020 Jul 1;12(7):1207-1216. doi: 10.1093/gbe/evaa122. Genome Biol Evol. 2020. PMID: 32544224 Free PMC article.
-
Chimeric inheritance and crown-group acquisitions of carbon fixation genes within Chlorobiales: Origins of autotrophy in Chlorobiales and implication for geological biomarkers.PLoS One. 2022 Oct 13;17(10):e0275539. doi: 10.1371/journal.pone.0275539. eCollection 2022. PLoS One. 2022. PMID: 36227849 Free PMC article.
-
Metabolic cross-feeding interactions modulate the dynamic community structure in microbial fuel cell under variable organic loading wastewaters.PLoS Comput Biol. 2024 Oct 17;20(10):e1012533. doi: 10.1371/journal.pcbi.1012533. eCollection 2024 Oct. PLoS Comput Biol. 2024. PMID: 39418284 Free PMC article.
-
Photoautotrophic Growth Rate Enhancement of Synechocystis sp. PCC6803 by Heterologous Production of 2-Oxoglutarate:Ferredoxin Oxidoreductase from Chlorobaculum tepidum.Biology (Basel). 2022 Dec 29;12(1):59. doi: 10.3390/biology12010059. Biology (Basel). 2022. PMID: 36671751 Free PMC article.
References
-
- Imhoff J. F., Thiel V. (2010) Photosynth. Res. 104, 123–136 - PubMed
-
- Overmann J. (2006) The Prokaryotes 3rd Ed., Vol. 7, pp. 359–378, Springer, New York
-
- Wahlund T. M., Woese C. R., Castenholz R. W., Madigan M. T. (1991) Arch. Microbiol. 156, 81–90
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

