The carboxy-terminal fragment of inhibitor-2 of protein phosphatase-2A induces Alzheimer disease pathology and cognitive impairment
- PMID: 20651003
- PMCID: PMC3229424
- DOI: 10.1096/fj.10-158477
The carboxy-terminal fragment of inhibitor-2 of protein phosphatase-2A induces Alzheimer disease pathology and cognitive impairment
Abstract
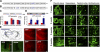
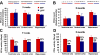
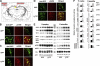
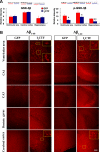
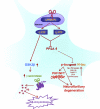
Development of rational therapeutic treatments of Alzheimer disease (AD) requires the elucidation of the etiopathogenic mechanisms of neurofibrillary degeneration and β-amyloidosis, the two hallmarks of this disease. Here we show, employing an adeno-associated virus serotype 1 (AAV1)-induced expression of the C-terminal fragment (I(2CTF)) of I(2)(PP2A), also called SET, in rat brain, decrease in protein phosphatase 2A (PP2A) activity, abnormal hyperphosphorylation of tau, and neurodegeneration; littermates treated identically but with vector only, i.e., AAV1-enhanced green fluorescent protein (GFP), served as a control. Furthermore, there was an increase in the level of activated glycogen synthase kinase-3β and enhanced expression of intraneuronal Aβ in AAV1-I(2CTF) animals. Morris water maze behavioral test revealed that infection with AAV1-I(2CTF) induced spatial reference memory and memory consolidation deficits and a decrease in the brain level of pSer133-CREB. These findings suggest a novel etiopathogenic mechanism of AD, which is initiated by the cleavage of I(2)(PP2A), producing I(2CTF), and describe a novel disease-relevant nontransgenic animal model of AD.
Figures

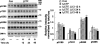
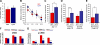
References
-
- Iqbal K., Flory M., Khatoon S., Soininen H., Pirttila T., Lehtovirta M., Alafuzoff I., Blennow K., Andreasen N., Vanmechelen E., Grundke-Iqbal I. (2005) Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann. Neurol. 58, 748– 757 - PubMed
-
- Alafuzoff I., Iqbal K., Friden H., Adolfsson R., Winblad B. (1987) Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol. (Berl.). 74, 209– 225 - PubMed
-
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 261, 6084– 6089 - PubMed
-
- Bennecib M., Gong C. X., Grundke-Iqbal I., Iqbal K. (2000) Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 485, 87– 93 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

