Origins, evolution, and phenotypic impact of new genes
- PMID: 20651121
- PMCID: PMC2945180
- DOI: 10.1101/gr.101386.109
Origins, evolution, and phenotypic impact of new genes
Abstract
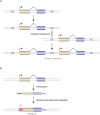
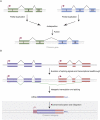
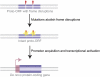
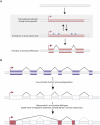
Ever since the pre-molecular era, the birth of new genes with novel functions has been considered to be a major contributor to adaptive evolutionary innovation. Here, I review the origin and evolution of new genes and their functions in eukaryotes, an area of research that has made rapid progress in the past decade thanks to the genomics revolution. Indeed, recent work has provided initial whole-genome views of the different types of new genes for a large number of different organisms. The array of mechanisms underlying the origin of new genes is compelling, extending way beyond the traditionally well-studied source of gene duplication. Thus, it was shown that novel genes also regularly arose from messenger RNAs of ancestral genes, protein-coding genes metamorphosed into new RNA genes, genomic parasites were co-opted as new genes, and that both protein and RNA genes were composed from scratch (i.e., from previously nonfunctional sequences). These mechanisms then also contributed to the formation of numerous novel chimeric gene structures. Detailed functional investigations uncovered different evolutionary pathways that led to the emergence of novel functions from these newly minted sequences and, with respect to animals, attributed a potentially important role to one specific tissue--the testis--in the process of gene birth. Remarkably, these studies also demonstrated that novel genes of the various types significantly impacted the evolution of cellular, physiological, morphological, behavioral, and reproductive phenotypic traits. Consequently, it is now firmly established that new genes have indeed been major contributors to the origin of adaptive evolutionary novelties.
Figures

References
-
- Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, Schwartz S, Adams MD, Myers EW, Li PW, Eichler EE 2002. Recent segmental duplications in the human genome. Science 297: 1003–1007 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
