Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2
- PMID: 20651288
- PMCID: PMC2941538
- DOI: 10.1161/CIRCRESAHA.109.215715
Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2
Abstract
Rationale: Macrophages change their phenotype and biological functions depending on the microenvironment. In atherosclerosis, oxidative tissue damage accompanies chronic inflammation; however, macrophage phenotypic changes in response to oxidatively modified molecules are not known.
Objective: To examine macrophage phenotypic changes in response to oxidized phospholipids that are present in atherosclerotic lesions.
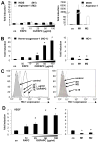
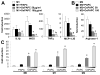
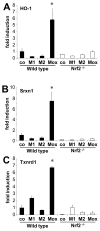
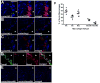
Methods and results: We show that oxidized phospholipid-treated murine macrophages develop into a novel phenotype (Mox) that is strikingly different from the conventional M1 and M2 macrophage phenotypes. Compared to M1 and M2, Mox macrophages show a different gene expression pattern, as well as decreased phagocytotic and chemotactic capacity. Treatment with oxidized phospholipids induces both M1 and M2 macrophages to switch to the Mox phenotype. Whole-genome expression array analysis and subsequent gene ontology clustering revealed that the Mox phenotype was characterized by abundant overrepresentation of Nrf2-mediated expression of redox-regulatory genes. In macrophages isolated from Nrf2(-/-) mice, oxidized phospholipid-induced gene expression and regulation of redox status were compromised. Moreover, we found that Mox macrophages comprise 30% of all macrophages in advanced atherosclerotic lesions of low-density lipoprotein receptor knockout (LDLR(-/-)) mice.
Conclusions: Together, we identify Nrf2 as a key regulator in the formation of a novel macrophage phenotype (Mox) that develops in response to oxidative tissue damage. The unique biological properties of Mox macrophages suggest this phenotype may play an important role in atherosclerotic lesion development as well as in other settings of chronic inflammation.
Figures


References
-
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005 December;5(12):953–64. - PubMed
-
- Martinez FO, Helming L, Gordon S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu Rev Immunol. 2008 December 23; - PubMed
-
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004 December;25(12):677–86. - PubMed
-
- Mantovani A, Muzio M, Garlanda C, Sozzani S, Allavena P. Macrophage control of inflammation: negative pathways of regulation of inflammatory cytokines. Novartis Found Symp. 2001;234:120–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

