O-GlcNAc signaling in the cardiovascular system
- PMID: 20651294
- PMCID: PMC2919351
- DOI: 10.1161/CIRCRESAHA.110.224675
O-GlcNAc signaling in the cardiovascular system
Abstract
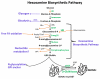
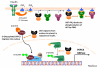
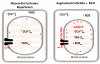
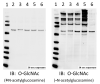
Cardiovascular function is regulated at multiple levels. Some of the most important aspects of such regulation involve alterations in an ever-growing list of posttranslational modifications. One such modification orchestrates input from numerous metabolic cues to modify proteins and alter their localization and/or function. Known as the beta-O-linkage of N-acetylglucosamine (ie, O-GlcNAc) to cellular proteins, this unique monosaccharide is involved in a diverse array of physiological and pathological functions. This review introduces readers to the general concepts related to O-GlcNAc, the regulation of this modification, and its role in primary pathophysiology. Much of the existing literature regarding the role of O-GlcNAcylation in disease addresses the protracted elevations in O-GlcNAcylation observed during diabetes. In this review, we focus on the emerging evidence of its involvement in the cardiovascular system. In particular, we highlight evidence of protein O-GlcNAcylation as an autoprotective alarm or stress response. We discuss recent literature supporting the idea that promoting O-GlcNAcylation improves cell survival during acute stress (eg, hypoxia, ischemia, oxidative stress), whereas limiting O-GlcNAcylation exacerbates cell damage in similar models. In addition to addressing the potential mechanisms of O-GlcNAc-mediated cardioprotection, we discuss technical issues related to studying protein O-GlcNAcylation in biological systems. The reader should gain an understanding of what protein O-GlcNAcylation is and that its roles in the acute and chronic disease settings appear distinct.
Figures
Similar articles
-
O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress.Biochim Biophys Acta. 2010 Feb;1800(2):96-106. doi: 10.1016/j.bbagen.2009.07.018. Epub 2009 Aug 6. Biochim Biophys Acta. 2010. PMID: 19647786 Free PMC article. Review.
-
O-GlcNAcylation and oxidation of proteins: is signalling in the cardiovascular system becoming sweeter?Clin Sci (Lond). 2012 Oct;123(8):473-86. doi: 10.1042/CS20110638. Clin Sci (Lond). 2012. PMID: 22757958 Free PMC article. Review.
-
O-GlcNAcylation and cardiovascular disease.Biochem Soc Trans. 2017 Apr 15;45(2):545-553. doi: 10.1042/BST20160164. Biochem Soc Trans. 2017. PMID: 28408494 Free PMC article. Review.
-
O-GlcNAc and the cardiovascular system.Pharmacol Ther. 2014 Apr;142(1):62-71. doi: 10.1016/j.pharmthera.2013.11.005. Epub 2013 Nov 25. Pharmacol Ther. 2014. PMID: 24287310 Free PMC article. Review.
-
Modification of histones by sugar β-N-acetylglucosamine (GlcNAc) occurs on multiple residues, including histone H3 serine 10, and is cell cycle-regulated.J Biol Chem. 2011 Oct 28;286(43):37483-95. doi: 10.1074/jbc.M111.284885. Epub 2011 Sep 6. J Biol Chem. 2011. PMID: 21896475 Free PMC article.
Cited by
-
Hyperglycemic memory in diabetic cardiomyopathy.Front Med. 2022 Feb;16(1):25-38. doi: 10.1007/s11684-021-0881-2. Epub 2021 Dec 18. Front Med. 2022. PMID: 34921674 Review.
-
Finding the missing link between the unfolded protein response and O-GlcNAcylation in the heart.Circ Res. 2014 Aug 29;115(6):546-8. doi: 10.1161/CIRCRESAHA.114.304855. Circ Res. 2014. PMID: 25170091 Free PMC article.
-
Impact of protein O-GlcNAcylation on neural tube malformation in diabetic embryopathy.Sci Rep. 2017 Sep 11;7(1):11107. doi: 10.1038/s41598-017-11655-6. Sci Rep. 2017. PMID: 28894244 Free PMC article.
-
The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism.Front Chem. 2020 Nov 26;8:592688. doi: 10.3389/fchem.2020.592688. eCollection 2020. Front Chem. 2020. PMID: 33330380 Free PMC article. Review.
-
Chemical tools to explore nutrient-driven O-GlcNAc cycling.Crit Rev Biochem Mol Biol. 2014 Jul-Aug;49(4):327-42. doi: 10.3109/10409238.2014.931338. Crit Rev Biochem Mol Biol. 2014. PMID: 25039763 Free PMC article. Review.
References
-
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of glycobiology. 2009:784. - PubMed
-
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. - PubMed
-
- Paterson AJ, Kudlow JE. Regulation of glutamine:Fructose-6-phosphate amidotransferase gene transcription by epidermal growth factor and glucose. Endocrinology. 1995;136:2809–2816. - PubMed
-
- Chang Q, Su K, Baker JR, Yang X, Paterson AJ, Kudlow JE. Phosphorylation of human glutamine:Fructose-6-phosphate amidotransferase by camp-dependent protein kinase at serine 205 blocks the enzyme activity. J. Biol. Chem. 2000;275:21981–21987. - PubMed
-
- Hu Y, Riesland L, Paterson AJ, Kudlow JE. Phosphorylation of mouse glutamine-fructose-6-phosphate amidotransferase 2 (gfat2) by camp-dependent protein kinase increases the enzyme activity. J Biol Chem. 2004;279:29988–29993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

