Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals
- PMID: 20651585
- PMCID: PMC2954261
- DOI: 10.1097/QAD.0b013e32833dba03
Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals
Abstract
Background: HAART can effectively reduce plasma HIV RNA levels to below the level of detection in most HIV-infected patients. The degree to which residual low-level viremia persists during HAART remains unclear.
Methods: We identified 180 individuals (median duration of HIV infection 12 years) who had at least two consecutive plasma HIV-1 RNA levels below the level of detection (<50-75 copies/ml) while taking antiretroviral drugs; 36 of 180 had been virologically suppressed for more than 5 years. Longitudinal plasma samples that were taken from these individuals during periods of viral load suppression were selected and analyzed. The isothermal transcription-mediated amplification (TMA) (limit of detection <3.5 copies RNA/ml) assay was used to measure persistent viremia. A 'detuned' EIA assay was used to obtain quantitative HIV antibody levels.
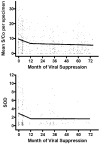
Results: A total of 1606 TMA assays were performed on 438 specimens in 180 HAART-suppressed individuals (median 3 replicates per specimen). In the first year of viral suppression, plasma RNA levels declined significantly (P = 0.001), but after month 12 there was no evidence for a continued decline (P = 0.383). In the first year of viral suppression, HIV antibody levels also declined (P = 0.054), but after month 12 there was no evidence for a continued decline (P = 0.988).
Conclusion: Viremia continued to decline during the first 12 months after viremia became undetectable using conventional methods, and then remained stable. HIV antibody levels also decreased in the first year of viral suppression and then remained stable. Viremia and the HIV-associated host response appear to achieve a steady-state 'set-point' during long-term combination therapy.
Conflict of interest statement
Figures

References
-
- Stramer SL, Glynn SA, Kleinman SH, Strong DM, Caglioti S, Wright DJ, et al. Detection of HIV-1 and HCV infections among antibody-negative blood donors by nucleic acid-amplification testing. N Engl J Med. 2004;351:760–768. - PubMed
-
- Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV-RNA testing in men who have sex with men. Aids. 2005;19:1323–1325. - PubMed
-
- Lelie PN, van Drimmelen HA, Cuypers HT, Best SJ, Stramer SL, Hyland C, et al. Sensitivity of HCV RNA and HIV RNA blood screening assays. Transfusion. 2002;42:527–536. - PubMed
-
- Busch MP, Hecht FM. Nucleic acid amplification testing for diagnosis of acute HIV infection: has the time come? Aids. 2005;19:1317–1319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 MH062246/MH/NIMH NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- R21 AI055273/AI/NIAID NIH HHS/United States
- K24 RR016482/RR/NCRR NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- R01 AI052745/AI/NIAID NIH HHS/United States
- P30 MH59037/MH/NIMH NIH HHS/United States
- 5-MO1-RR00083-37/RR/NCRR NIH HHS/United States
- K23 AI075985/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01-AI067854/AI/NIAID NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
- P30 MH62246/MH/NIMH NIH HHS/United States
- P30 AI27763/AI/NIAID NIH HHS/United States
- AI052745/AI/NIAID NIH HHS/United States
- RR16482/RR/NCRR NIH HHS/United States
- K23AI075985/AI/NIAID NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- AI055273/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

