Targeting the dynamic HSP90 complex in cancer
- PMID: 20651736
- PMCID: PMC6778733
- DOI: 10.1038/nrc2887
Targeting the dynamic HSP90 complex in cancer
Abstract
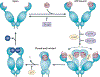
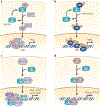
The molecular chaperone heat shock protein 90 (HSP90) has been used by cancer cells to facilitate the function of numerous oncoproteins, and it can be argued that cancer cells are 'addicted' to HSP90. However, although recent reports of the early clinical efficacy of HSP90 inhibitors are encouraging, the optimal use of HSP90-targeted therapeutics will depend on understanding the complexity of HSP90 regulation and the degree to which HSP90 participates in both neoplastic and normal cellular physiology.
Figures

References
-
- Wandinger SK, Richter K. & Buchner J. The Hsp90 chaperone machinery. J. Biol. Chem. 283, 18473–18477 (2008). - PubMed
-
- Zhao R. et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120, 715–727 (2005). - PubMed
-
- Pratt WB & Toft DO Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. (Maywood) 228, 111–133 (2003). - PubMed
-
- Whitesell L. & Lindquist SL HSP90 and the chaperoning of cancer. Nature Rev. Cancer 5, 761–772 (2005). - PubMed
-
- Dezwaan DC & Freeman BC HSP90: the Rosetta stone for cellular protein dynamics? Cell Cycle 7, 1006–1012 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

