Prostate cancer as a model for tumour immunotherapy
- PMID: 20651745
- PMCID: PMC3082366
- DOI: 10.1038/nri2817
Prostate cancer as a model for tumour immunotherapy
Abstract
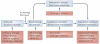
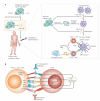
Advances in basic immunology have led to an improved understanding of the interactions between the immune system and tumours, generating renewed interest in approaches that aim to treat cancer immunologically. As clinical and preclinical studies of tumour immunotherapy illustrate several immunological principles, a review of these data is broadly instructive and is particularly timely now that several agents are beginning to show evidence of efficacy. This is especially relevant in the case of prostate cancer, as recent approval of sipuleucel-T by the US Food and Drug Administration marks the first antigen-specific immunotherapy approved for cancer treatment. Although this Review focuses on immunotherapy for prostate cancer, the principles discussed are applicable to many tumour types, and the approaches discussed are highlighted in that context.
Figures

References
-
- Stat. bite: estimated worldwide cancer mortality among men, 2002. J. Natl Cancer Inst. 2005;97:1402. [No authors listed] - PubMed
-
- Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N. Engl. J. Med. 2007;357:2696–2705. - PubMed
-
- Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. - PubMed
-
- Petrylak DP, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004;351:1513–1520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

