Synthesis of high coercivity core-shell nanorods based on nickel and cobalt and their magnetic properties
- PMID: 20651915
- PMCID: PMC2893701
- DOI: 10.1007/s11671-009-9459-7
Synthesis of high coercivity core-shell nanorods based on nickel and cobalt and their magnetic properties
Abstract
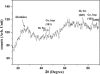
Hybrid magnetic nanostructures with high coercivity have immense application potential in various fields. Nickel (Ni) electrodeposited inside Cobalt (Co) nanotubes (a new system named Ni @ Co nanorods) were fabricated using a two-step potentiostatic electrodeposition method. Ni @ Co nanorods were crystalline, and they have an average diameter of 150 nm and length of ~15 μm. The X-ray diffraction studies revealed the existence of two separate phases corresponding to Ni and Co. Ni @ Co nanorods exhibited a very high longitudinal coercivity. The general mobility-assisted growth mechanism proposed for the growth of one-dimensional nanostructures inside nano porous alumina during potentiostatic electrodeposition is found to be valid in this case too.
Keywords: Core–shell nanostructures; Hybrid nanostructures; Magnetic nanowires; Mobility-assisted growth mechanism; Nanorods.
Figures




References
-
- Narayanan TN, Shaijumon MM, Ajayan PM, Anantharaman MR. J. Phys. Chem. C. 2008. p. 14281. COI number [1:CAS:528:DC%2BD1cXhtValurnF] - DOI
-
- Iijima S. Nature. 1991. p. 56. COI number [1:CAS:528:DyaK38Xmt1Ojtg%3D%3D]; Bibcode number [1991Natur.354...56I] - DOI
-
- Bao J, Tie C, Xu Z, Zhou Q, Shen D, Ma Q. Adv. 2001. p. 21. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

