SULFANYLATION OF 1,3-DITHIANE ANIONS BY 5-(ALKYLSULFANYL)-1-PHENYLTETRAZOLES
- PMID: 20651936
- PMCID: PMC2907546
- DOI: 10.1135/cccc2008195
SULFANYLATION OF 1,3-DITHIANE ANIONS BY 5-(ALKYLSULFANYL)-1-PHENYLTETRAZOLES
Abstract
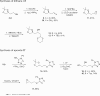
An unusual reaction between 1,3-dithiane anions and by 5-(alkylsulfanyl)-1-phenyltetrazoles has been discovered in which the dithiane anion formally displaces the 1-phenyltetrazole ring from sulfur to provide a sulfanylated dithiane.
Figures

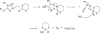


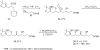
References
-
- Kobayashi Y, Czechtizky W, Kishi Y. Org. Lett. 2003;5:93. - PubMed
-
- BouzBouz S, Cossy J. Org. Lett. 2004;6:3469. - PubMed
-
- Gudipati V. Ph.D. Thesis. University of Pittsburgh; Pittsburgh: 2008. http://etd.library.pitt.edu/ETD/available/etd-04212008-115104/. The thesis contains copies of key spectra of 19 and other compounds.
Grants and funding
LinkOut - more resources
Full Text Sources
