Proprotein convertase inhibition results in decreased skin cell proliferation, tumorigenesis, and metastasis
- PMID: 20651981
- PMCID: PMC2907578
- DOI: 10.1593/neo.92030
Proprotein convertase inhibition results in decreased skin cell proliferation, tumorigenesis, and metastasis
Abstract
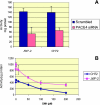
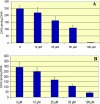
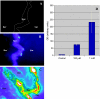
PACE4 is a proprotein convertase (PC) responsible for cleaving and activating proteins that contribute to enhance tumor progression. PACE4 overexpression significantly increased the susceptibility to carcinogenesis, leading to enhanced tumor cell proliferation and premature degradation of the basement membrane. In the present study, we sought to evaluate a novel approach to retard skin tumor progression based on the inhibition of PACE4. We used decanoyl-RVKR-chloromethylketone (CMK), a small-molecule PC inhibitor, for in vitro and in vivo experiments. We found that CMK-dependent blockage of PACE4 activity in skin squamous cell carcinoma cell lines resulted in impaired insulin-like growth factor 1 receptor maturation, diminished its intrinsic tyrosine kinase activity, and decreased tumor cell proliferation. Two-stage skin chemical carcinogenesis experiments, together with topical applications of CMK, demonstrated that this PC inhibitor markedly reduced tumor incidence, tumor multiplicity, and metastasis, pointing to a significant delay in tumor progression in wild-type and PACE4 transgenic mice. These results identify PACE4, together with other PCs, as suitable targets to slow down or block tumor progression, suggesting that PC inhibition is a potential approach for therapy for solid tumors.
Figures







Similar articles
-
Enhanced aggressiveness of benzopyrene-induced squamous carcinomas in transgenic mice overexpressing the proprotein convertase PACE4 (PCSK6).Mol Carcinog. 2015 Oct;54(10):1122-31. doi: 10.1002/mc.22183. Epub 2014 May 21. Mol Carcinog. 2015. PMID: 24845697 Free PMC article.
-
Inhibition of the prohormone convertase subtilisin-kexin isoenzyme-1 induces apoptosis in human melanoma cells.J Invest Dermatol. 2014 Jan;134(1):168-175. doi: 10.1038/jid.2013.282. Epub 2013 Jun 27. J Invest Dermatol. 2014. PMID: 23884247
-
Enhanced UV-induced skin carcinogenesis in transgenic mice overexpressing proprotein convertases.Neoplasia. 2013 Feb;15(2):169-79. doi: 10.1593/neo.121846. Neoplasia. 2013. PMID: 23441131 Free PMC article.
-
Proprotein convertase inhibition: Paralyzing the cell's master switches.Biochem Pharmacol. 2017 Sep 15;140:8-15. doi: 10.1016/j.bcp.2017.04.027. Epub 2017 Apr 27. Biochem Pharmacol. 2017. PMID: 28456517 Free PMC article. Review.
-
Inhibition of proprotein convertases: approaches to block squamous carcinoma development and progression.Mol Carcinog. 2007 Aug;46(8):654-9. doi: 10.1002/mc.20331. Mol Carcinog. 2007. PMID: 17440928 Review.
Cited by
-
Opposite roles of furin and PC5A in N-cadherin processing.Neoplasia. 2012 Oct;14(10):880-92. doi: 10.1593/neo.121250. Neoplasia. 2012. PMID: 23097623 Free PMC article.
-
The Role of Proprotein Convertases in the Regulation of the Function of Immune Cells in the Oncoimmune Response.Front Immunol. 2021 Apr 29;12:667850. doi: 10.3389/fimmu.2021.667850. eCollection 2021. Front Immunol. 2021. PMID: 33995401 Free PMC article. Review.
-
Dinosaurs and ancient civilizations: reflections on the treatment of cancer.Neoplasia. 2010 Dec;12(12):957-68. doi: 10.1593/neo.101588. Neoplasia. 2010. PMID: 21170260 Free PMC article.
-
Transgenic overexpression of the proprotein convertase furin enhances skin tumor growth.Neoplasia. 2012 Apr;14(4):271-82. doi: 10.1593/neo.12166. Neoplasia. 2012. PMID: 22577343 Free PMC article.
-
The interconnectedness of cancer cell signaling.Neoplasia. 2011 Dec;13(12):1183-93. doi: 10.1593/neo.111746. Neoplasia. 2011. PMID: 22241964 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
