Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha
- PMID: 20651983
- PMCID: PMC2907580
- DOI: 10.1593/neo.92106
Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha
Abstract
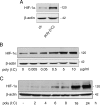
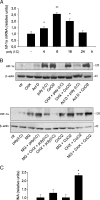
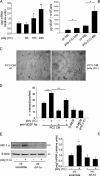
Toll-like receptors (TLRs) recognize microbial/viral-derived components that trigger innate immune response and conflicting data implicate TLR agonists in cancer, either as protumor or antitumor agents. We previously demonstrated that TLR3 activation mediated by its agonist poly(I:C) induces antitumor signaling, leading to apoptosis of prostate cancer cells LNCaP and PC3 with much more efficiency in the former than in the second more aggressive line. The transcription factor hypoxia-inducible factor 1 (HIF-1) regulates several cellular processes, including apoptosis, in response to hypoxia and to other stimuli also in normoxic conditions. Here we describe a novel protumor machinery triggered by TLR3 activation in PC3 cells consisting of increased expression of the specific I.3 isoform of HIF-1 alpha and nuclear accumulation of HIF-1 complex in normoxia, resulting in reduced apoptosis and in secretion of functional vascular endothelial growth factor (VEGF). Moreover, we report that, in the less aggressive LNCaP cells, TLR3 activation fails to induce nuclear accumulation of HIF-1 alpha. However, the transfection of I.3 isoform of hif-1 alpha in LNCaP cells allows poly(I:C)-induced HIF-1 activation, resulting in apoptosis protection and VEGF secretion. Altogether, our findings demonstrate that differences in the basal level of HIF-1 alpha expression in different prostate cancer cell lines underlie their differential response to TLR3 activation, suggesting a correlation between different stages of malignancy, hypoxic gene expression, and beneficial responsiveness to TLR agonists.
Figures



Similar articles
-
Aromatic Hydrocarbon Receptor Suppresses Prostate Cancer Bone Metastasis Cells-Induced Vasculogenesis of Endothelial Progenitor Cells under Hypoxia.Cell Physiol Biochem. 2016;39(2):709-20. doi: 10.1159/000445662. Epub 2016 Jul 25. Cell Physiol Biochem. 2016. PMID: 27448695
-
Rhapontigenin inhibited hypoxia inducible factor 1 alpha accumulation and angiogenesis in hypoxic PC-3 prostate cancer cells.Biol Pharm Bull. 2011;34(6):850-5. doi: 10.1248/bpb.34.850. Biol Pharm Bull. 2011. PMID: 21628883
-
Peroxiredoxin 1 stimulates endothelial cell expression of VEGF via TLR4 dependent activation of HIF-1α.PLoS One. 2012;7(11):e50394. doi: 10.1371/journal.pone.0050394. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185615 Free PMC article.
-
The Role of Hypoxia-inducible Factor-1 in Bladder Cancer.Curr Mol Med. 2024;24(7):827-834. doi: 10.2174/1566524023666230720163448. Curr Mol Med. 2024. PMID: 37475553 Free PMC article. Review.
-
Hypoxic Modulation of HLA-G Expression through the Metabolic Sensor HIF-1 in Human Cancer Cells.J Immunol Res. 2017;2017:4587520. doi: 10.1155/2017/4587520. Epub 2017 Jul 11. J Immunol Res. 2017. PMID: 28781970 Free PMC article. Review.
Cited by
-
Toll-like receptors in prostate infection and cancer between bench and bedside.J Cell Mol Med. 2013 Jun;17(6):713-22. doi: 10.1111/jcmm.12055. Epub 2013 Apr 4. J Cell Mol Med. 2013. PMID: 23551576 Free PMC article. Review.
-
Exploiting poly(I:C) to induce cancer cell apoptosis.Cancer Biol Ther. 2017 Oct 3;18(10):747-756. doi: 10.1080/15384047.2017.1373220. Epub 2017 Sep 7. Cancer Biol Ther. 2017. PMID: 28881163 Free PMC article. Review.
-
Soluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edema.Curr Diab Rep. 2013 Aug;13(4):476-80. doi: 10.1007/s11892-013-0382-z. Curr Diab Rep. 2013. PMID: 23649946 Free PMC article. Review.
-
Hypoxia as a Modulator of Inflammation and Immune Response in Cancer.Cancers (Basel). 2022 May 4;14(9):2291. doi: 10.3390/cancers14092291. Cancers (Basel). 2022. PMID: 35565420 Free PMC article. Review.
-
Bufalin Suppresses Migration and Invasion of Hepatocellular Carcinoma Cells Elicited by Poly (I:C) Therapy.Oncoimmunology. 2018 Feb 8;7(5):e1426434. doi: 10.1080/2162402X.2018.1426434. eCollection 2018. Oncoimmunology. 2018. PMID: 29721392 Free PMC article.
References
-
- Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. - PubMed
-
- Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009;122:1055–1057. - PubMed
-
- Depping R, Hagele S, Wagner KF, Wiesner RJ, Camenisch G, Wenger RH, Katschinski DM. A dominant-negative isoform of hypoxia-inducible factor-1α specifically expressed in human testis. Biol Reprod. 2004;71:331–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
