How do bisphosphonates inhibit bone metastasis in vivo?
- PMID: 20651986
- PMCID: PMC2907583
- DOI: 10.1593/neo.10282
How do bisphosphonates inhibit bone metastasis in vivo?
Abstract
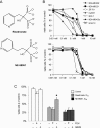
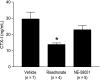
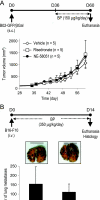
Bisphosphonates are potent inhibitors of osteoclast-mediated bone resorption and have demonstrated clinical utility in the treatment of patients with osteolytic bone metastases. They also exhibit direct antitumor activity in vitro and can reduce skeletal tumor burden and inhibit the formation of bone metastases in vivo. However, whether such effects are caused by a direct action of bisphosphonates on tumor cells or indirectly through inhibition of bone resorption remains unclear. To address this question, we used here a structural analog of the bisphosphonate risedronate, NE-58051, which has a bone mineral affinity similar to that of risedronate, but a 3000-fold lower bone antiresorptive activity. In vitro, risedronate and NE-58051 inhibited proliferation of breast cancer and melanoma cell lines. In vivo, risedronate and NE-58051 did not inhibit the growth of subcutaneous B02 breast tumor xenografts or the formation of B16F10 melanoma lung metastasis. In contrast to NE-58051, risedronate did inhibit B02 breast cancer bone metastasis formation by reducing both bone destruction and skeletal tumor burden, indicating that the antitumor effect of bisphosphonates is achieved mainly through inhibition of osteoclast-mediated bone resorption.
Figures

Similar articles
-
Lowering bone mineral affinity of bisphosphonates as a therapeutic strategy to optimize skeletal tumor growth inhibition in vivo.Cancer Res. 2008 Nov 1;68(21):8945-53. doi: 10.1158/0008-5472.CAN-08-2195. Cancer Res. 2008. PMID: 18974139
-
Cancer-induced bone loss and associated pain-related behavior is reduced by risedronate but not its phosphonocarboxylate analog NE-10790.Int J Cancer. 2009 Sep 1;125(5):1177-85. doi: 10.1002/ijc.24436. Int J Cancer. 2009. PMID: 19444917
-
Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice.Cancer Res. 1995 Aug 15;55(16):3551-7. Cancer Res. 1995. PMID: 7627963
-
Oral bisphosphonates: A review of clinical use in patients with bone metastases.Cancer. 2000 Jan 1;88(1):6-14. Cancer. 2000. PMID: 10618600 Review.
-
[Bone targeting agents: bisphosphonates].Bull Cancer. 2013 Nov;100(11):1199-206. doi: 10.1684/bdc.2013.1840. Bull Cancer. 2013. PMID: 24158668 Review. French.
Cited by
-
Optimal management of cancer treatment-induced bone loss: considerations for elderly patients.Drugs Aging. 2011 Nov 1;28(11):867-83. doi: 10.2165/11595820-000000000-00000. Drugs Aging. 2011. PMID: 22054228 Review.
-
Bisphosphonates as anticancer agents in early breast cancer: preclinical and clinical evidence.Breast Cancer Res. 2015 Sep 2;17(1):121. doi: 10.1186/s13058-015-0634-8. Breast Cancer Res. 2015. PMID: 26328589 Free PMC article. Review.
-
From prostate to bone: key players in prostate cancer bone metastasis.Cancers (Basel). 2011;3(1):478-93. doi: 10.3390/cancers3010478. Cancers (Basel). 2011. PMID: 21603150 Free PMC article.
-
Dormancy in breast cancer.Breast Cancer (Dove Med Press). 2012 Dec 5;4:183-91. doi: 10.2147/BCTT.S26431. Breast Cancer (Dove Med Press). 2012. PMID: 24367205 Free PMC article. Review.
-
Bisphosphonates in the adjuvant treatment of young women with breast cancer: the estrogen rich is a poor candidate!J Thorac Dis. 2013 Jun;5 Suppl 1(Suppl 1):S27-35. doi: 10.3978/j.issn.2072-1439.2013.06.04. J Thorac Dis. 2013. PMID: 23819025 Free PMC article.
References
-
- Clezardin P, Ebetino FH, Fournier PGJ. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res. 2005;65:4971–4974. - PubMed
-
- Lipton A. Emerging role of bisphosphonates in the clinic—antitumor activity and prevention of metastasis to bone. Cancer Treat Rev. 2008;34(suppl 1):S25–S30. - PubMed
-
- Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crinò L, Dirix L, Gnant M, Gralow J, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19:420–432. - PubMed
-
- Coxon FP, Thompson K, Rogers MJ. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol. 2006;6:307–312. - PubMed
-
- Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
