Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination
- PMID: 20652006
- PMCID: PMC2905956
- DOI: 10.1155/2010/747892
Neuroinvasion in prion diseases: the roles of ascending neural infection and blood dissemination
Abstract
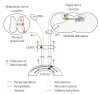
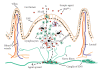
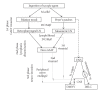
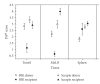
Prion disorders are infectious, neurodegenerative diseases that affect humans and animals. Susceptibility to some prion diseases such as kuru or the new variant of Creutzfeldt-Jakob disease in humans and scrapie in sheep and goats is influenced by polymorphisms of the coding region of the prion protein gene, while other prion disorders such as fatal familial insomnia, familial Creutzfeldt-Jakob disease, or Gerstmann-Straussler-Scheinker disease in humans have an underlying inherited genetic basis. Several prion strains have been demonstrated experimentally in rodents and sheep. The progression and pathogenesis of disease is influenced by both genetic differences in the prion protein and prion strain. Some prion diseases only affect the central nervous system whereas others involve the peripheral organs prior to neuroinvasion. Many experiments undertaken in different species and using different prion strains have postulated common pathways of neuroinvasion. It is suggested that prions access the autonomic nerves innervating peripheral organs and tissues to finally reach the central nervous system. We review here published data supporting this view and additional data suggesting that neuroinvasion may concurrently or independently involve the blood vascular system.
Figures





Similar articles
-
Human transmissible spongiform encephalopathies: historic view.Handb Clin Neurol. 2018;153:1-17. doi: 10.1016/B978-0-444-63945-5.00001-5. Handb Clin Neurol. 2018. PMID: 29887130 Review.
-
[Prion diseases or transmissible spongiform encephalopathies].Rev Med Interne. 2022 Feb;43(2):106-115. doi: 10.1016/j.revmed.2021.05.002. Epub 2021 Jun 18. Rev Med Interne. 2022. PMID: 34148672 French.
-
Prion diseases.Handb Clin Neurol. 2017;145:393-403. doi: 10.1016/B978-0-12-802395-2.00028-6. Handb Clin Neurol. 2017. PMID: 28987186 Review.
-
How an Infection of Sheep Revealed Prion Mechanisms in Alzheimer's Disease and Other Neurodegenerative Disorders.Int J Mol Sci. 2021 May 4;22(9):4861. doi: 10.3390/ijms22094861. Int J Mol Sci. 2021. PMID: 34064393 Free PMC article. Review.
-
Neurodegeneration in humans caused by prions.West J Med. 1994 Sep;161(3):264-72. West J Med. 1994. PMID: 7975565 Free PMC article. Review.
Cited by
-
Prions efficiently cross the intestinal barrier after oral administration: Study of the bioavailability, and cellular and tissue distribution in vivo.Sci Rep. 2016 Aug 30;6:32338. doi: 10.1038/srep32338. Sci Rep. 2016. PMID: 27573341 Free PMC article.
-
Chronic wasting disease: a cervid prion infection looming to spillover.Vet Res. 2021 Sep 6;52(1):115. doi: 10.1186/s13567-021-00986-y. Vet Res. 2021. PMID: 34488900 Free PMC article. Review.
-
Permeability of the windows of the brain: feasibility of dynamic contrast-enhanced MRI of the circumventricular organs.Fluids Barriers CNS. 2020 Oct 28;17(1):66. doi: 10.1186/s12987-020-00228-x. Fluids Barriers CNS. 2020. PMID: 33115484 Free PMC article. Clinical Trial.
-
Neuroinvasion of α-Synuclein Prionoids after Intraperitoneal and Intraglossal Inoculation.J Virol. 2016 Sep 29;90(20):9182-93. doi: 10.1128/JVI.01399-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489279 Free PMC article.
-
Experimental H-type bovine spongiform encephalopathy characterized by plaques and glial- and stellate-type prion protein deposits.Vet Res. 2011 Jun 23;42(1):79. doi: 10.1186/1297-9716-42-79. Vet Res. 2011. PMID: 21699704 Free PMC article.
References
-
- Fraser H. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature. 1982;295(5845):149–150. - PubMed
-
- Scott JR, Fraser H. Transport and targeting of scrapie infectivity and pathology in the optic nerve projections following intraocular infection. Progress in Clinical and Biological Research. 1989;317:645–652. - PubMed
-
- Sbriccoli M, Cardone F, Valanzano A, et al. Neuroinvasion of the 263 K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathologica. 2009;117(2):175–184. - PubMed
LinkOut - more resources
Full Text Sources

