Engineering a biological pacemaker: in vivo, in vitro and in silico models
- PMID: 20652091
- PMCID: PMC2905832
- DOI: 10.1016/j.ddmod.2009.06.001
Engineering a biological pacemaker: in vivo, in vitro and in silico models
Abstract
Several hundred thousand electronic pacemakers are implanted in the US each year to treat abnormally slow heart rates. Biological pacemaker research strives to replace this hardware, and the associated monitoring and maintenance, by using gene or cell therapy to create a permanent and autonomically responsive pacemaker. While there are numerous technological hurdles to overcome before this is a therapeutic reality, one critical issue is determining the optimal channel gene to employ in creating a biological pacemaker. This review discusses the pros and cons of various model systems for characterizing and evaluating the function of candidate channel genes. It is argued that a sequential approach that combines in silico, in vitro and in vivo models is required.
Figures
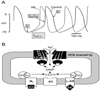
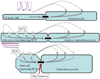
Similar articles
-
Permanent pacemakers in older persons.J Am Geriatr Soc. 1999 Sep;47(9):1125-35. doi: 10.1111/j.1532-5415.1999.tb05239.x. J Am Geriatr Soc. 1999. PMID: 10484258 Review.
-
HCN2/SkM1 gene transfer into canine left bundle branch induces stable, autonomically responsive biological pacing at physiological heart rates.J Am Coll Cardiol. 2013 Mar 19;61(11):1192-201. doi: 10.1016/j.jacc.2012.12.031. Epub 2013 Feb 6. J Am Coll Cardiol. 2013. PMID: 23395072 Free PMC article.
-
Biventricular pacing (cardiac resynchronization therapy): an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5(13):1-60. Epub 2005 Sep 1. Ont Health Technol Assess Ser. 2005. PMID: 23074464 Free PMC article.
-
Harnessing cell reprogramming for cardiac biological pacing.J Biomed Sci. 2023 Aug 26;30(1):74. doi: 10.1186/s12929-023-00970-y. J Biomed Sci. 2023. PMID: 37633890 Free PMC article. Review.
-
Stem cell-based biological pacemakers from proof of principle to therapy: a review.Cytotherapy. 2014 Jul;16(7):873-80. doi: 10.1016/j.jcyt.2014.02.014. Epub 2014 May 13. Cytotherapy. 2014. PMID: 24831844 Free PMC article. Review.
Cited by
-
HCN channels and heart rate.Molecules. 2012 Apr 5;17(4):4225-35. doi: 10.3390/molecules17044225. Molecules. 2012. PMID: 22481543 Free PMC article. Review.
-
Current research trends and challenges in tissue engineering for mending broken hearts.Life Sci. 2019 Jul 15;229:233-250. doi: 10.1016/j.lfs.2019.05.012. Epub 2019 May 17. Life Sci. 2019. PMID: 31103607 Free PMC article. Review.
References
-
- Rosen MR, et al. Cardiac pacing: from biological to electronic … to biological? Circulation Arrhythmia Electrophysiology. 2008;1:54–61. - PubMed
-
- Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm. 2009;6(5):605–610. - PubMed
-
- Robinson RB, et al. I(f) and the biological pacemaker. Pharmacol.Res. 2006;53(5):407–415. - PubMed
-
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Ann.Rev.Physiol. 2003;65:453–480. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
