Efficacy of catumaxomab in tumor spheroid killing is mediated by its trifunctional mode of action
- PMID: 20652245
- PMCID: PMC11030560
- DOI: 10.1007/s00262-010-0894-1
Efficacy of catumaxomab in tumor spheroid killing is mediated by its trifunctional mode of action
Abstract
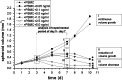
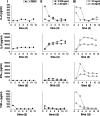
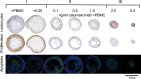
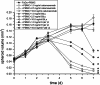
Catumaxomab is an intact trifunctional bispecific antibody targeting human EpCAM (epithelial cell adhesion molecule) and CD3 with further binding to Fcgamma receptor type I, IIa and III. We choose multicellular tumor spheroids (MCTS) of human EpCAM-positive FaDu tumor cells in co-culture with human peripheral blood mononuclear cells as an adequate three-dimensional in vitro model for pharmacological testing of catumaxomab. We found a strong dose-dependent antitumor response mediated by catumaxomab, with volume-decreased or completely destroyed tumor spheroids together with a massive immune cell infiltration and decreased signals for cancer cell viability and clonogenicity. In control experiments with F(ab')2 fragments of catumaxomab and the parental antibodies alone or in combination the effects in spheroid volume reduction were less than that of catumaxomab. All binding partners of the postulated tricell complex have to be present to exert catumaxomab's full mode of action. These distinct effects of catumaxomab are based on the unique composition of the trifunctional bispecific antibody. Since, in general, many cancers are treated by chemotherapy in combination with immunological tumor therapy, we additionally analyzed the effects of cisplatin alone and in combination with catumaxomab. For cisplatin alone we detected a dose-dependent response relating to decrease of spheroid volume. The combined approach resulted in a synergistic spheroid volume decrease and the colony formation was reduced to non-detectable levels.
Figures
Similar articles
-
Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy.Cancer Treat Rev. 2010 Oct;36(6):458-67. doi: 10.1016/j.ctrv.2010.03.001. Epub 2010 Mar 27. Cancer Treat Rev. 2010. PMID: 20347527 Review.
-
Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): a phase I study.Cancer Immunol Immunother. 2007 Oct;56(10):1637-44. doi: 10.1007/s00262-007-0310-7. Epub 2007 Apr 5. Cancer Immunol Immunother. 2007. PMID: 17410361 Free PMC article. Clinical Trial.
-
Test system for trifunctional antibodies in 3D MCTS culture.J Biomol Screen. 2009 Sep;14(8):980-90. doi: 10.1177/1087057109341766. Epub 2009 Aug 12. J Biomol Screen. 2009. PMID: 19675312
-
Transient lymphocyte decrease due to adhesion and migration following catumaxomab (anti-EpCAM x anti-CD3) treatment in vivo.Clin Transl Oncol. 2012 May;14(5):376-81. doi: 10.1007/s12094-012-0811-5. Clin Transl Oncol. 2012. PMID: 22551544
-
Catumaxomab: clinical development and future directions.MAbs. 2010 Mar-Apr;2(2):129-36. doi: 10.4161/mabs.2.2.11221. MAbs. 2010. PMID: 20190561 Free PMC article. Review.
Cited by
-
Validation of a Three-Dimensional Head and Neck Spheroid Model to Evaluate Cameras for NIR Fluorescence-Guided Cancer Surgery.Int J Mol Sci. 2021 Feb 17;22(4):1966. doi: 10.3390/ijms22041966. Int J Mol Sci. 2021. PMID: 33671198 Free PMC article.
-
Cytotoxicity and infiltration of human NK cells in in vivo-like tumor spheroids.BMC Cancer. 2015 May 3;15:351. doi: 10.1186/s12885-015-1321-y. BMC Cancer. 2015. PMID: 25933805 Free PMC article.
-
A Bispecific Antibody-Based Approach for Targeting Mesothelin in Triple Negative Breast Cancer.Front Immunol. 2019 Jul 10;10:1593. doi: 10.3389/fimmu.2019.01593. eCollection 2019. Front Immunol. 2019. PMID: 31354732 Free PMC article.
-
Suppression of c-Met-Overexpressing Tumors by a Novel c-Met/CD3 Bispecific Antibody.Drug Des Devel Ther. 2020 Aug 7;14:3201-3214. doi: 10.2147/DDDT.S254117. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32982167 Free PMC article.
-
Recent advances and challenges of bispecific antibodies in solid tumors.Exp Hematol Oncol. 2021 Dec 18;10(1):56. doi: 10.1186/s40164-021-00250-1. Exp Hematol Oncol. 2021. PMID: 34922633 Free PMC article. Review.
References
-
- Zeidler R, Mysliwietz J, Csanady M, Walz A, Ziegler I, Schmitt B, Wollenberg B, Lindhofer H. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer. 2000;83:261–266. doi: 10.1054/bjoc.2000.1237. - DOI - PMC - PubMed
-
- Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, Lindhofer H. Simultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killing. J Immunol. 1999;163:1246–1252. - PubMed
-
- Riesenberg R, Buchner A, Pohla H, Lindhofer H. Lysis of prostate carcinoma cells by trifunctional bispecific antibodies (alpha EpCAM × alpha CD3) J Histochem Cytochem. 2001;49:911–917. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

