Function of Nanos2 in the male germ cell lineage in mice
- PMID: 20652721
- PMCID: PMC11115876
- DOI: 10.1007/s00018-010-0456-x
Function of Nanos2 in the male germ cell lineage in mice
Abstract
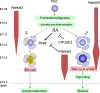
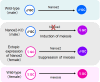
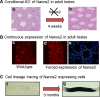
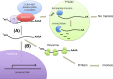
Nanos is known as an evolutionarily conserved RNA-binding protein, the function of which is implicated in germ cell development. This includes the maintenance of both the primordial germ cells (PGCs) and germline stem cells. In mice, Nanos2 exhibits a unique feature in which its expression is induced only in the germ cells within the sexually determined male gonad. Nanos2 promotes male germ cell differentiation, while simultaneously suppressing a female program. In addition, Nanos2 is also expressed in the spermatogonial stem cells and functions as an intrinsic factor to maintain the stem cell population during spermatogenesis. Detailed cytological and biochemical analyses in embryonic male gonads in the mouse have revealed that Nanos2 localizes to the P-bodies, a center of RNA processing. It has also been shown that the Nanos2 interacts with protein components of the deadenylation complex involved in the initial step of the RNA degradation pathway.
Figures
Similar articles
-
Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells.J Cell Sci. 2010 Mar 15;123(Pt 6):871-80. doi: 10.1242/jcs.057968. Epub 2010 Feb 16. J Cell Sci. 2010. PMID: 20159962
-
Expression and intracellular localization of Nanos2-homologue protein in primordial germ cells and spermatogonial stem cells.Zygote. 2019 Apr;27(2):82-88. doi: 10.1017/S0967199419000066. Epub 2019 Mar 19. Zygote. 2019. PMID: 30888312
-
Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development.Development. 2007 Jan;134(1):77-83. doi: 10.1242/dev.02697. Epub 2006 Nov 30. Development. 2007. PMID: 17138666
-
[Nanos2 in the male reproductive system: Progress in studies].Zhonghua Nan Ke Xue. 2018 Jun;24(6):558-561. Zhonghua Nan Ke Xue. 2018. PMID: 30173464 Review. Chinese.
-
Mouse germ cell development during embryogenesis.Curr Opin Genet Dev. 2008 Aug;18(4):337-41. doi: 10.1016/j.gde.2008.06.003. Epub 2008 Jul 28. Curr Opin Genet Dev. 2008. PMID: 18625315 Review.
Cited by
-
Mechanisms of Hormonal, Genetic, and Temperature Regulation of Germ Cell Proliferation, Differentiation, and Death During Spermatogenesis.Biomolecules. 2025 Mar 29;15(4):500. doi: 10.3390/biom15040500. Biomolecules. 2025. PMID: 40305231 Free PMC article. Review.
-
LncRNA HOTAIR promotes proliferation and suppresses apoptosis of mouse spermatogonium GC-1 cells by sponging miR-761 to modulate NANOS2 expression.In Vitro Cell Dev Biol Anim. 2022 Apr;58(4):295-306. doi: 10.1007/s11626-022-00657-y. Epub 2022 Apr 14. In Vitro Cell Dev Biol Anim. 2022. PMID: 35426065
-
Molecular Cloning and Sexually Dimorphic Expression Analysis of nanos2 in the Sea Urchin, Mesocentrotus nudus.Int J Mol Sci. 2019 Jun 1;20(11):2705. doi: 10.3390/ijms20112705. Int J Mol Sci. 2019. PMID: 31159444 Free PMC article.
-
Regulatory mechanism of protein metabolic pathway during the differentiation process of chicken male germ cell.In Vitro Cell Dev Biol Anim. 2015 Aug;51(7):655-61. doi: 10.1007/s11626-015-9877-z. Epub 2015 Mar 21. In Vitro Cell Dev Biol Anim. 2015. PMID: 25794557
-
The RNA-binding protein Adad1 is necessary for germ cell maintenance and meiosis in zebrafish.PLoS Genet. 2023 Aug 8;19(8):e1010589. doi: 10.1371/journal.pgen.1010589. eCollection 2023 Aug. PLoS Genet. 2023. PMID: 37552671 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

