Translating Sleeping Beauty transposition into cellular therapies: victories and challenges
- PMID: 20652893
- PMCID: PMC3971908
- DOI: 10.1002/bies.201000027
Translating Sleeping Beauty transposition into cellular therapies: victories and challenges
Erratum in
- Bioessays. 2011 Jun;33(6):478-9
Abstract
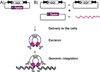
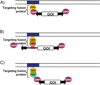
Recent results confirm that long-term expression of therapeutic transgenes can be achieved by using a transposon-based system in primary stem cells and in vivo. Transposable elements are natural DNA transfer vehicles that are capable of efficient genomic insertion. The latest generation, Sleeping Beauty transposon-based hyperactive vector (SB100X), is able to address the basic problem of non-viral approaches - that is, low efficiency of stable gene transfer. The combination of transposon-based non-viral gene transfer with the latest improvements of non-viral delivery techniques could provide a long-term therapeutic effect without compromising biosafety. The new challenges of pre-clinical research will focus on further refinement of the technology in large animal models and improving the safety profile of SB vectors by target-selected transgene integration into genomic "safe harbors." The first clinical application of the SB system will help to validate the safety of this approach.
Figures



References
-
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. - PubMed
-
- Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. - PubMed
-
- Riviere C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. - PubMed
-
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

