FRS2α regulates Erk levels to control a self-renewal target Hes1 and proliferation of FGF-responsive neural stem/progenitor cells
- PMID: 20652960
- PMCID: PMC2996081
- DOI: 10.1002/stem.488
FRS2α regulates Erk levels to control a self-renewal target Hes1 and proliferation of FGF-responsive neural stem/progenitor cells
Abstract
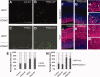
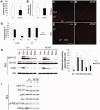
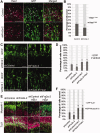
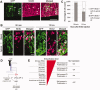
Fibroblast growth factor (FGF) is among the most common growth factors used in cultures to maintain self-renewal and proliferative capabilities of a variety of stem cells, including neural stem cells (NSCs). However, the molecular mechanisms underlying the control by FGF have remained elusive. Studies on mutant mice of FGF receptor substrate 2α (FRS2α), a central mediator for FGF signaling, combined with FRS2α knockdown or gain-of-function experiments, allowed us to dissect the role of FGF signaling for the self-renewal and proliferation of NSCs and to provide novel molecular mechanisms for them. We identified Hes1 as a novel self-renewal target of FGF-signaling. Quantitatively different levels of Erk activation mediated by FRS2α may regulate self-renewal of NSCs and proliferation of neural stem/progenitor cells (NSPCs); low levels of Erk activation are sufficient for the former, however, higher levels are required for maximum activity of the latter. Thus, FRS2α fine-tunes the FGF-signaling to control qualitatively different biological activities, self-renewal at least partly through Hes1 versus proliferation of NSPCs.
Figures



Similar articles
-
An FGF4-FRS2alpha-Cdx2 axis in trophoblast stem cells induces Bmp4 to regulate proper growth of early mouse embryos.Stem Cells. 2010 Jan;28(1):113-21. doi: 10.1002/stem.247. Stem Cells. 2010. PMID: 19890878
-
Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells.Mol Cell Biol. 2008 Dec;28(24):7427-41. doi: 10.1128/MCB.01962-07. Epub 2008 Oct 13. Mol Cell Biol. 2008. PMID: 18852283 Free PMC article.
-
Fibroblast growth factor receptor 1 (FGFR1) tyrosine phosphorylation regulates binding of FGFR substrate 2alpha (FRS2alpha) but not FRS2 to the receptor.Mol Endocrinol. 2008 Jan;22(1):167-75. doi: 10.1210/me.2007-0140. Epub 2007 Sep 27. Mol Endocrinol. 2008. PMID: 17901128 Free PMC article.
-
Rhythmic gene expression in somite formation and neural development.Mol Cells. 2009 May 31;27(5):497-502. doi: 10.1007/s10059-009-0068-1. Epub 2009 May 15. Mol Cells. 2009. PMID: 19466597 Review.
-
Control of stemness by fibroblast growth factor signaling in stem cells and cancer stem cells.Curr Stem Cell Res Ther. 2009 Jan;4(1):9-15. doi: 10.2174/157488809787169048. Curr Stem Cell Res Ther. 2009. PMID: 19149625 Review.
Cited by
-
Hes1 triggers epithelial-mesenchymal transition (EMT)-like cellular marker alterations and promotes invasion and metastasis of nasopharyngeal carcinoma by activating the PTEN/AKT pathway.Oncotarget. 2015 Nov 3;6(34):36713-30. doi: 10.18632/oncotarget.5457. Oncotarget. 2015. PMID: 26452025 Free PMC article.
-
Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy.J Clin Invest. 2015 Jun;125(6):2484-96. doi: 10.1172/JCI74883. Epub 2015 May 11. J Clin Invest. 2015. PMID: 25961456 Free PMC article.
-
An autocrine/paracrine circuit of growth differentiation factor (GDF) 15 has a role for maintenance of breast cancer stem-like cells.Oncotarget. 2017 Apr 11;8(15):24869-24881. doi: 10.18632/oncotarget.15276. Oncotarget. 2017. PMID: 28206960 Free PMC article.
-
Crosstalk of Intercellular Signaling Pathways in the Generation of Midbrain Dopaminergic Neurons In Vivo and from Stem Cells.J Dev Biol. 2019 Jan 15;7(1):3. doi: 10.3390/jdb7010003. J Dev Biol. 2019. PMID: 30650592 Free PMC article. Review.
-
EphA4 Regulates the Balance between Self-Renewal and Differentiation of Radial Glial Cells and Intermediate Neuronal Precursors in Cooperation with FGF Signaling.PLoS One. 2015 May 15;10(5):e0126942. doi: 10.1371/journal.pone.0126942. eCollection 2015. PLoS One. 2015. PMID: 25978062 Free PMC article.
References
-
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. - PubMed
-
- Yeoh JS, de Haan G. Fibroblast growth factors as regulators of stem cell self-renewal and aging. Mech Ageing Dev. 2007;128:17–24. - PubMed
-
- Gotoh N. Control of stemness by fibroblast growth factor signaling in stem cells and cancer stem cells. Curr Stem Cell Res Ther. 2009;4:9–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

