Association of carboxylesterase 1A genotypes with irinotecan pharmacokinetics in Japanese cancer patients
- PMID: 20653675
- PMCID: PMC2911552
- DOI: 10.1111/j.1365-2125.2010.03695.x
Association of carboxylesterase 1A genotypes with irinotecan pharmacokinetics in Japanese cancer patients
Abstract
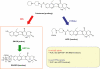
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT * Association of UDP-glucuronosyltransferase 1A1 (UGT1A1) genetic polymorphisms *6 and *28 with reduced clearance of SN-38 and severe neutropenia in irinotecan therapy was demonstrated in Japanese cancer patients. * The detailed gene structure of CES1 has been characterized. * Possible functional SNPs in the promoter region have been reported. WHAT THIS STUDY ADDS * Association of functional CES1 gene number with AUC ratio [(SN-38 + SN-38G)/irinotecan], an in vivo index of CES activity, was observed in patients with irinotecan monotherapy. * No significant effects of major CES1 SNPs on irinotecan PK were detected. AIMS Human carboxylesterase 1 (CES1) hydrolyzes irinotecan to produce an active metabolite SN-38 in the liver. The human CES1 gene family consists of two functional genes, CES1A1 (1A1) and CES1A2 (1A2), which are located tail-to-tail on chromosome 16q13-q22.1 (CES1A2-1A1). The pseudogene CES1A3 (1A3) and a chimeric CES1A1 variant (var1A1) are also found as polymorphic isoforms of 1A2 and 1A1, respectively. In this study, roles of CES1 genotypes and major SNPs in irinotecan pharmacokinetics were investigated in Japanese cancer patients. METHODS CES1A diplotypes [combinations of haplotypes A (1A3-1A1), B (1A2-1A1), C (1A3-var1A1) and D (1A2-var1A1)] and the major SNPs (-75T>G and -30G>A in 1A1, and -816A>C in 1A2 and 1A3) were determined in 177 Japanese cancer patients. Associations of CES1 genotypes, number of functional CES1 genes (1A1, 1A2 and var1A1) and major SNPs, with the AUC ratio of (SN-38 + SN-38G)/irinotecan, a parameter of in vivo CES activity, were analyzed for 58 patients treated with irinotecan monotherapy. RESULTS The median AUC ratio of patients having three or four functional CES1 genes (diplotypes A/B, A/D or B/C, C/D, B/B and B/D; n= 35) was 1.24-fold of that in patients with two functional CES1 genes (diplotypes A/A, A/C and C/C; n= 23) [median (25th-75th percentiles): 0.31 (0.25-0.38) vs. 0.25 (0.20-0.32), P= 0.0134]. No significant effects of var1A1 and the major SNPs examined were observed. CONCLUSION This study suggests a gene-dose effect of functional CES1A genes on SN-38 formation in irinotecan-treated Japanese cancer patients.
Figures



References
-
- Humerickhouse R, Lohrbach K, Li L, Bosron WF, Dolan ME. Characterization of CPT-11 hydrolysis by human liver carboxylesterase isoforms hCE-1 and hCE-2. Cancer Res. 2000;60:1189–92. - PubMed
-
- Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60:6921–6. - PubMed
-
- Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ. UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J. 2002;2:43–7. - PubMed
-
- Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramírez J, Rudin CM, Vokes EE, Ratain MJ. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. 2004;22:1382–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

