Dismantling of Arabidopsis thaliana mesophyll cell chloroplasts during natural leaf senescence
- PMID: 20653883
- PMCID: PMC4383266
- DOI: 10.1111/j.1438-8677.2009.00206.x
Dismantling of Arabidopsis thaliana mesophyll cell chloroplasts during natural leaf senescence
Abstract
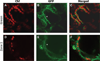
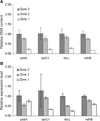
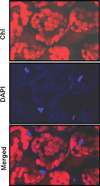
One of the earliest events in the process of leaf senescence is dismantling of chloroplasts. Mesophyll cell chloroplasts from rosette leaves were studied in Arabidopsis thaliana undergoing natural senescence. The number of chloroplasts decreased by only 17% in fully yellow leaves, and chloroplasts were found to undergo progressive photosynthetic and ultrastructural changes as senescence proceeded. In ultrastructural studies, an intact tonoplast could not be visualized, thus, a 35S-GFP::delta-TIP line with a GFP-labeled tonoplast was used to demonstrate that chloroplasts remain outside of the tonoplast even at late stages of senescence. Chloroplast DNA was measured by real-time PCR at four different chloroplast loci, and a fourfold decrease in chloroplast DNA per chloroplast was noted in yellow senescent leaves when compared to green leaves from plants of the same age. Although chloroplast DNA did decrease, the chloroplast/nuclear gene copy ratio was still 31:1 in yellow leaves. Interestingly, mRNA levels for the four loci differed: psbA and ndhB mRNAs remained abundant late into senescence, while rpoC1 and rbcL mRNAs decreased in parallel to chloroplast DNA. Together, these data demonstrate that, during senescence, chloroplasts remain outside of the vacuole as distinct organelles while the thylakoid membranes are dismantled internally. As thylakoids were dismantled, Rubisco large subunit, Lhcb1, and chloroplast DNA levels declined, but variable levels of mRNA persisted.
Figures




References
-
- Barton R. Fine structure of mesophyll cells in senescing leaves of Phaseolus. Planta. 1966;71:314–325. - PubMed
-
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin J-F, Wu S-H, Swidzinski J, Ishizaki K, Leaver CJ. Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark / starvation- induced senescence in Arabidopsis. Plant Journal. 2005;42:567–585. - PubMed
-
- Chiba A, Ishida H, Nishizawa NK, Makino A, Mae T. Exclusion of ribulose-1,5-bisphosphate carboxylase / oxygenase from chloroplasts by specific bodies in naturally senescing leaves of wheat. Plant and Cell Physiology. 2003;44:914–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

