Host lung gene expression patterns predict infectious etiology in a mouse model of pneumonia
- PMID: 20653947
- PMCID: PMC2914038
- DOI: 10.1186/1465-9921-11-101
Host lung gene expression patterns predict infectious etiology in a mouse model of pneumonia
Abstract
Background: Lower respiratory tract infections continue to exact unacceptable worldwide mortality, often because the infecting pathogen cannot be identified. The respiratory epithelia provide protection from pneumonias through organism-specific generation of antimicrobial products, offering potential insight into the identity of infecting pathogens. This study assesses the capacity of the host gene expression response to infection to predict the presence and identity of lower respiratory pathogens without reliance on culture data.
Methods: Mice were inhalationally challenged with S. pneumoniae, P. aeruginosa, A. fumigatus or saline prior to whole genome gene expression microarray analysis of their pulmonary parenchyma. Characteristic gene expression patterns for each condition were identified, allowing the derivation of prediction rules for each pathogen. After confirming the predictive capacity of gene expression data in blinded challenges, a computerized algorithm was devised to predict the infectious conditions of subsequent subjects.
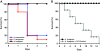
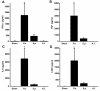
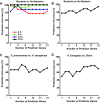
Results: We observed robust, pathogen-specific gene expression patterns as early as 2 h after infection. Use of an algorithmic decision tree revealed 94.4% diagnostic accuracy when discerning the presence of bacterial infection. The model subsequently differentiated between bacterial pathogens with 71.4% accuracy and between non-bacterial conditions with 70.0% accuracy, both far exceeding the expected diagnostic yield of standard culture-based bronchoscopy with bronchoalveolar lavage.
Conclusions: These data substantiate the specificity of the pulmonary innate immune response and support the feasibility of a gene expression-based clinical tool for pneumonia diagnosis.
Figures


References
-
- WHO. The World Health Report 2004 -- Changing History. Geneva: World Health Organization; 2004.
-
- Rano A, Agusti C, Jimenez P, Angrill J, Benito N, Danes C, Gonzalez J, Rovira M, Pumarola T, Moreno A. et al. Pulmonary infiltrates in non-HIV immunocompromised patients: a diagnostic approach using non-invasive and bronchoscopic procedures. Thorax. 2001;56(5):379–387. doi: 10.1136/thorax.56.5.379. - DOI - PMC - PubMed
-
- Bissinger AL, Einsele H, Hamprecht K, Schumacher U, Kandolf R, Loeffler J, Aepinus C, Bock T, Jahn G, Hebart H. Infectious pulmonary complications after stem cell transplantation or chemotherapy: diagnostic yield of bronchoalveolar lavage. Diagn Microbiol Infect Dis. 2005;52(4):275–280. doi: 10.1016/j.diagmicrobio.2005.03.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

