A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development
- PMID: 20653957
- PMCID: PMC2914679
- DOI: 10.1186/1471-213X-10-76
A novel transgenic zebrafish model for blood-brain and blood-retinal barrier development
Abstract
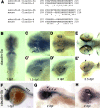
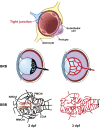
Background: Development and maintenance of the blood-brain and blood-retinal barrier is critical for the homeostasis of brain and retinal tissue. Despite decades of research our knowledge of the formation and maintenance of the blood-brain (BBB) and blood-retinal (BRB) barrier is very limited. We have established an in vivo model to study the development and maintenance of these barriers by generating a transgenic zebrafish line that expresses a vitamin D-binding protein fused with enhanced green fluorescent protein (DBP-EGFP) in blood plasma, as an endogenous tracer.
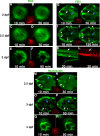
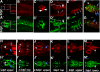
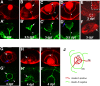
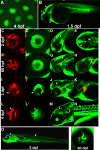
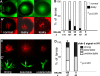
Results: The temporal establishment of the BBB and BRB was examined using this transgenic line and the results were compared with that obtained by injection of fluorescent dyes into the sinus venosus of embryos at various stages of development. We also examined the expression of claudin-5, a component of tight junctions during the first 4 days of development. We observed that the BBB of zebrafish starts to develop by 3 dpf, with expression of claudin-5 in the central arteries preceding it at 2 dpf. The hyaloid vasculature in the zebrafish retina develops a barrier function at 3 dpf, which endows the zebrafish with unique advantages for studying the BRB.
Conclusion: Zebrafish embryos develop BBB and BRB function simultaneously by 3 dpf, which is regulated by tight junction proteins. The Tg(l-fabp:DBP-EGFP) zebrafish will have great advantages in studying development and maintenance of the blood-neural barrier, which is a new application for the widely used vertebrate model.
Figures
Similar articles
-
Retinoic acid signaling is essential for maintenance of the blood-retinal barrier.FASEB J. 2018 Oct;32(10):5674-5684. doi: 10.1096/fj.201701469R. Epub 2018 Jun 6. FASEB J. 2018. PMID: 29874129 Free PMC article.
-
Investigation of barrier characteristics in the hyaloid-retinal vessel of zebrafish.J Neurosci Res. 2011 Jun;89(6):921-8. doi: 10.1002/jnr.22607. Epub 2011 Mar 15. J Neurosci Res. 2011. PMID: 21412815
-
Primary cilia are present on endothelial cells of the hyaloid vasculature but are not required for the development of the blood-retinal barrier.PLoS One. 2020 Jul 31;15(7):e0225351. doi: 10.1371/journal.pone.0225351. eCollection 2020. PLoS One. 2020. PMID: 32735563 Free PMC article.
-
Claudin-5a in developing zebrafish brain barriers: another brick in the wall.Bioessays. 2010 Sep;32(9):768-76. doi: 10.1002/bies.201000045. Bioessays. 2010. PMID: 20652895 Review.
-
Involvement of claudins in zebrafish brain ventricle morphogenesis.Ann N Y Acad Sci. 2012 Jun;1257:193-8. doi: 10.1111/j.1749-6632.2012.06507.x. Ann N Y Acad Sci. 2012. PMID: 22671606 Review.
Cited by
-
A Fluorescence-Based Assay for Proteinuria Screening in Larval Zebrafish (Danio rerio).Zebrafish. 2015 Oct;12(5):372-6. doi: 10.1089/zeb.2015.1093. Epub 2015 Jun 30. Zebrafish. 2015. PMID: 26125680 Free PMC article.
-
A distinct mechanism of vascular lumen formation in Xenopus requires EGFL7.PLoS One. 2015 Feb 23;10(2):e0116086. doi: 10.1371/journal.pone.0116086. eCollection 2015. PLoS One. 2015. PMID: 25705891 Free PMC article.
-
Developmental Neurotoxicity of the Harmful Algal Bloom Toxin Domoic Acid: Cellular and Molecular Mechanisms Underlying Altered Behavior in the Zebrafish Model.Environ Health Perspect. 2020 Nov;128(11):117002. doi: 10.1289/EHP6652. Epub 2020 Nov 4. Environ Health Perspect. 2020. PMID: 33147070 Free PMC article.
-
Zebrafish glial-vascular interactions progressively expand over the course of brain development.iScience. 2024 Dec 9;28(1):111549. doi: 10.1016/j.isci.2024.111549. eCollection 2025 Jan 17. iScience. 2024. PMID: 39811646 Free PMC article.
-
Fatty Acid-Binding Protein 4-Mediated Regulation Is Pivotally Involved in Retinal Pathophysiology: A Review.Int J Mol Sci. 2024 Jul 14;25(14):7717. doi: 10.3390/ijms25147717. Int J Mol Sci. 2024. PMID: 39062961 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

