Cinnamon extract induces tumor cell death through inhibition of NFkappaB and AP1
- PMID: 20653974
- PMCID: PMC2920880
- DOI: 10.1186/1471-2407-10-392
Cinnamon extract induces tumor cell death through inhibition of NFkappaB and AP1
Erratum in
-
Correction to: Cinnamon extract induces tumor cell death through inhibition of NFκB and AP1.BMC Cancer. 2019 Nov 14;19(1):1113. doi: 10.1186/s12885-019-6342-5. BMC Cancer. 2019. PMID: 31727003 Free PMC article.
Abstract
Background: Cinnamomum cassia bark is the outer skin of an evergreen tall tree belonging to the family Lauraceae containing several active components such as essential oils (cinnamic aldehyde and cinnamyl aldehyde), tannin, mucus and carbohydrate. They have various biological functions including anti-oxidant, anti-microbial, anti-inflammation, anti-diabetic and anti-tumor activity. Previously, we have reported that anti-cancer effect of cinnamon extracts is associated with modulation of angiogenesis and effector function of CD8+ T cells. In this study, we further identified that anti-tumor effect of cinnamon extracts is also link with enhanced pro-apoptotic activity by inhibiting the activities NFkappaB and AP1 in mouse melanoma model.
Methods: Water soluble cinnamon extract was obtained and quality of cinnamon extract was evaluated by HPLC (High Performance Liquid Chromatography) analysis. In this study, we tested anti-tumor activity and elucidated action mechanism of cinnamon extract using various types of tumor cell lines including lymphoma, melanoma, cervix cancer and colorectal cancer in vitro and in vivo mouse melanoma model.
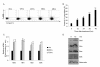
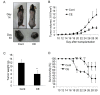
Results: Cinnamon extract strongly inhibited tumor cell proliferation in vitro and induced active cell death of tumor cells by up-regulating pro-apoptotic molecules while inhibiting NFkappaB and AP1 activity and their target genes such as Bcl-2, BcL-xL and survivin. Oral administration of cinnamon extract in melanoma transplantation model significantly inhibited tumor growth with the same mechanism of action observed in vitro.
Conclusion: Our study suggests that anti-tumor effect of cinnamon extracts is directly linked with enhanced pro-apoptotic activity and inhibition of NFkappaB and AP1 activities and their target genes in vitro and in vivo mouse melanoma model. Hence, further elucidation of active components of cinnamon extract could lead to development of potent anti-tumor agent or complementary and alternative medicine for the treatment of diverse cancers.
Figures

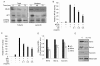

References
-
- Längler A, Kaatsch P, Spix C, Seifert G. Complementary and alternative treatment methods in children with cancer. A population based retrospective survey on the prevalence of use in Germany. European Journal of Integrative Medicine. 2008;1(Supplement 1):10. doi: 10.1016/j.eujim.2008.08.015. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

