Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage
- PMID: 20654597
- PMCID: PMC2933941
- DOI: 10.1016/j.brainres.2010.07.040
Luminal platelet aggregates in functional deficits in parenchymal vessels after subarachnoid hemorrhage
Abstract
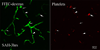
The pathophysiology of early ischemic injury after aneurysmal subarachnoid hemorrhage (SAH) is not understood. This study examined the acute effect of endovascular puncture-induced SAH on parenchymal vessel function in rat, using intravascular fluorescent tracers to assess flow and vascular permeability and immunostaining to assess structural integrity and to visualize platelet aggregates. In sham-operated animals, vessels were well filled with tracer administered 10s before sacrifice, and parenchymal escape of tracer was rare. At ten minutes and three hours after hemorrhage, patches of poor vascular filling were distributed throughout the forebrain. Close examination of these regions revealed short segments of narrowed diameter along many profiles. Most vascular profiles with reduced perfusion contained platelet aggregates and in addition showed focal loss of collagen IV, a principal component of basal lamina. In contrast, vessels were well filled at 24h post-hemorrhage, indicating that vascular perfusion had recovered. Parenchymal escape of intravascular tracer was detected at 10 min post-hemorrhage and later as plumes of fluorescence emanating into parenchyma from restricted microvascular foci. These data demonstrate that parenchymal microvessels are compromised in function by 10 min after SAH and identify focal microvascular constriction and local accumulation of luminal platelet aggregates as potential initiators of that compromise.
Copyright (c) 2010 Elsevier B.V. All rights reserved.
Figures







References
-
- Akopov S, Sercombe R, Seylaz J. Cerebrovascular reactivity: role of endothelium/platelet/leukocyte interactions. Cerebrovasc Brain Metab Rev. 1996;8:11–94. - PubMed
-
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. - PubMed
-
- Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins ALr, Vallabhajosyula P. Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery. 1998;42:352–360. - PubMed
-
- Doczi T. The pathogenetic and prognostic significance of blood-brain barrier damage at the acute stage of aneurysmal subarachnoid haemorrhage. Clinical and experimental studies. Acta Neurochir (Wien) 1985;77:110–132. - PubMed
-
- Doczi T, Joo F, Adam G, Bozoky B, Szerdahelyi P. Blood-brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery. 1986;18:733–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

