Probing the two-domain structure of homodimeric prokaryotic and eukaryotic catalase-peroxidases
- PMID: 20654740
- PMCID: PMC3513708
- DOI: 10.1016/j.bbapap.2010.07.013
Probing the two-domain structure of homodimeric prokaryotic and eukaryotic catalase-peroxidases
Abstract
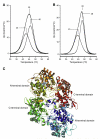
Catalase-peroxidases (KatGs) are ancestral bifunctional heme peroxidases found in archaeons, bacteria and lower eukaryotes. In contrast to homologous cytochrome c peroxidase (CcP) and ascorbate peroxidase (APx) homodimeric KatGs have a two-domain monomeric structure with a catalytic N-terminal heme domain and a C-terminal domain of high sequence and structural similarity but without obvious function. Nevertheless, without its C-terminal counterpart the N-terminal domain exhibits neither catalase nor peroxidase activity. Except some hybrid-type proteins all other members of the peroxidase-catalase superfamily lack this C-terminal domain. In order to probe the role of the two-domain monomeric structure for conformational and thermal stability urea and temperature-dependent unfolding experiments were performed by using UV-Vis-, electronic circular dichroism- and fluorescence spectroscopy, as well as differential scanning calorimetry. Recombinant prokaryotic (cyanobacterial KatG from Synechocystis sp. PCC6803) and eukaryotic (fungal KatG from Magnaporthe grisea) were investigated. The obtained data demonstrate that the conformational and thermal stability of bifunctional KatGs is significantly lower compared to homologous monofunctional peroxidases. The N- and C-terminal domains do not unfold independently. Differences between the cyanobacterial and the fungal enzyme are relatively small. Data will be discussed with respect to known structure and function of KatG, CcP and APx.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures





References
-
- Klotz M, Loewen PC. The molecular evolution of catalatic hydroperoxidases: evidence for multiple lateral transfer of genes between prokaryota and form bacteria into eukaryota. Mol. Biol. Evol. 2003;20:1098–1112. - PubMed
-
- Passardi F, Bakalovic N, Teixeira FK, Margis-Pinheiro M, Penel C, Dunand C. Prokaryotic origins of the non-animal peroxidase superfamily and organelle-mediated transmission to eukaryotes. Genomics. 2007;89:567–579. - PubMed
-
- Zamocky M, Obinger C. Molecular phylogeny of heme peroxidases. In: Ayala M, Torres E, editors. Biocatalysis Based on Heme Peroxidases. Springer; 2010. ISBN: 978-3-642-12626-0.
-
- Passardi F, Zamocky M, Favet J, Jakopitsch C, Penel C, Obinger C, Dunand C. Phylogenetic distribution of catalase-peroxidases: are there patches of order in chaos? Gene. 2007;397:101–113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

