Structural perspectives on secondary active transporters
- PMID: 20655602
- PMCID: PMC2933288
- DOI: 10.1016/j.tips.2010.06.004
Structural perspectives on secondary active transporters
Abstract
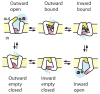
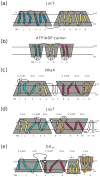
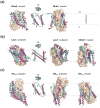
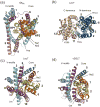
Secondary active transporters catalyze the concentrative transport of substrates across lipid membranes by harnessing the energy of electrochemical ion gradients. These transporters bind their ligands on one side of the membrane, and undergo a global conformational change to release them on the other side of the membrane. Over the last few years, crystal structures have captured several bacterial secondary transporters in different states along their transport cycle, providing insight into possible molecular mechanisms. In this review, we summarize recent findings focusing on the emerging structural and mechanistic similarities between evolutionary diverse transporters. We also discuss the structural basis of substrate binding, ion coupling and inhibition viewed from the perspective of these similarities.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Hediger MA, et al. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447:465–468. - PubMed
-
- Lolkema JS, Slotboom DJ. Classification of 29 families of secondary transport proteins into a single structural class using hydropathy profile analysis. J Mol Biol. 2003;327:901–909. - PubMed
-
- Brett CL, et al. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–239. - PubMed
-
- Lolkema JS, Slotboom DJ. The major amino acid transporter superfamily has a similar core structure as Na+-galactose and Na+-leucine transporters. Mol Membr Biol. 2008;25:567–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

