B-lymphocyte homeostasis and BLyS-directed immunotherapy in transplantation
- PMID: 20655723
- PMCID: PMC2946495
- DOI: 10.1016/j.trre.2010.05.004
B-lymphocyte homeostasis and BLyS-directed immunotherapy in transplantation
Abstract
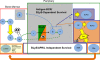
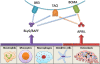
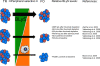
Current strategies for immunotherapy after transplantation are primarily T-lymphocyte directed and effectively abrogate acute rejection. However, the reality of chronic allograft rejection attests to the fact that transplantation tolerance remains an elusive goal. Donor-specific antibodies are considered the primary cause of chronic rejection. When naive, alloreactive B-cells encounter alloantigen and are activated, a resilient "sensitized" state, characterized by the presence of high-affinity antibody, is established. Here, we will delineate findings that support transient B-lymphocyte depletion therapy at the time of transplantation to preempt sensitization by eliminating alloreactive specificities from the recipient B-cell pool (ie, "repertoire remodeling"). Recent advances in our understanding of B-lymphocyte homeostasis provide novel targets for immunomodulation in transplantation. Specifically, the tumor necrosis factor-related cytokine BLyS is the dominant survival factor for "tolerance-susceptible" transitional and "preimmune" mature follicular B-cells. The transitional phenotype is the intermediate through which all newly formed B-cells pass before maturing into the follicular subset, which is responsible for mounting an alloantigen-specific antibody response. Systemic BLyS levels dictate the stringency of negative selection during peripheral B-cell repertoire development. Thus, targeting BLyS will likely provide an opportunity for repertoire-directed therapy to eliminate alloreactive B-cell specificities in transplant recipients, a requirement for the achievement of humoral tolerance and prevention of chronic rejection. In this review, the fundamentals of preimmune B-cell selection, homeostasis, and activation will be described. Furthermore, new and current B-lymphocyte-directed therapy for antibody-mediated rejection and the highly sensitized state will be discussed. Overall, our objective is to propose a rational approach for induction of humoral transplantation tolerance by remodeling the primary B-cell repertoire of the allograft recipient.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Murine islet allograft tolerance upon blockade of the B-lymphocyte stimulator, BLyS/BAFF.Transplantation. 2012 Apr 15;93(7):676-85. doi: 10.1097/TP.0b013e318246621d. Transplantation. 2012. PMID: 22262127
-
APRIL/BLyS Blockade Reduces Donor-specific Antibodies in Allosensitized Mice.Transplantation. 2019 Jul;103(7):1372-1384. doi: 10.1097/TP.0000000000002686. Transplantation. 2019. PMID: 30830041 Free PMC article.
-
Primary B cell repertoire remodeling to achieve humoral transplantation tolerance.Semin Immunol. 2012 Apr;24(2):109-14. doi: 10.1016/j.smim.2011.08.016. Epub 2011 Oct 5. Semin Immunol. 2012. PMID: 21978627 Review.
-
Essential role for B cells in transplantation tolerance.Curr Opin Immunol. 2011 Oct;23(5):685-91. doi: 10.1016/j.coi.2011.07.011. Curr Opin Immunol. 2011. PMID: 21982511 Free PMC article. Review.
-
Abrogation of antibody-mediated allograft rejection by regulatory CD4 T cells with indirect allospecificity.J Immunol. 2007 Feb 15;178(4):2221-8. doi: 10.4049/jimmunol.178.4.2221. J Immunol. 2007. PMID: 17277127
Cited by
-
Targeting B cells and antibody in transplantation.Am J Transplant. 2011 Jul;11(7):1359-67. doi: 10.1111/j.1600-6143.2011.03554.x. Epub 2011 Jun 10. Am J Transplant. 2011. PMID: 21668625 Free PMC article. Review.
-
Resolve, revise, and relax: the 3 Rs of B cell repertoire adjustment.Immunol Lett. 2012 Mar 30;143(1):2-8. doi: 10.1016/j.imlet.2012.01.014. Epub 2012 Feb 6. Immunol Lett. 2012. PMID: 22330846 Free PMC article. Review.
-
Serum Soluble B Cell-Activating Factor Is a Non-Invasive Biomarker of Antibody-Mediated Rejection in Kidney Allograft With Satisfactory Risk Stratification Performance But Negligible Diagnostic Value.Front Immunol. 2022 Apr 13;13:869444. doi: 10.3389/fimmu.2022.869444. eCollection 2022. Front Immunol. 2022. PMID: 35493478 Free PMC article.
-
B-Cell Activating Factor Predicts Acute Rejection Risk in Kidney Transplant Recipients: A 6-Month Follow-Up Study.Front Immunol. 2019 May 15;10:1046. doi: 10.3389/fimmu.2019.01046. eCollection 2019. Front Immunol. 2019. PMID: 31156628 Free PMC article.
-
Rational clinical trial design for antibody mediated renal allograft injury.Front Biosci (Landmark Ed). 2015 Jan 1;20(4):743-62. doi: 10.2741/4334. Front Biosci (Landmark Ed). 2015. PMID: 25553476 Free PMC article. Review.
References
-
- Terasaki PI, Cai J. Human leukocyte antigen antibodies and chronic rejection: from association to causation. Transplantation. 2008;86(3):377. - PubMed
-
- Colvin RB. Pathology of chronic humoral rejection. Contrib Nephrol. 2009;162:75. - PubMed
-
- Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5(10):807. - PubMed
-
- Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol. 2006;1(3):421. - PubMed
-
- Kirk AD, Baldwin WM, Cascalho MI, Chong AS, Sykes M, West LJ. American society of transplantation symposium on B cells in transplantation: harnessing humoral immunity from rodent models to clinical practice. Am J Transplant. 2007;7:1464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

