Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7)
- PMID: 20655807
- PMCID: PMC2918709
- DOI: 10.1016/j.transci.2010.05.014
Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7)
Abstract
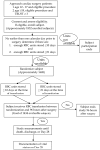
The question of whether storage of red blood cells (RBCs) alters their capacity to deliver oxygen and affects patient outcomes remains in a state of clinical equipoise. Studies of the changes which occur while RBCs are stored have led to several physiologically plausible hypotheses that these changes impair RBC function when the units are transfused. Although there is some evidence of this effect in vivo from animal model experiments, the results of several largely retrospective patient studies have not been consistent. Some studies have shown an association between worse clinical outcomes and transfusion of RBC which have been stored for longer periods of time, while others have found no effect. Three multicenter, randomized, controlled trials have been developed to address this important, but currently unanswered, question. Two clinical trials, one in low birth weight neonates and the other in intensive care unit patients, are enrolling subjects in Canada (the Age of Red Blood Cells in Premature Infants; the Age of Blood Study). The third trial, which is being developed in the United States, is the Red Cell Storage Duration Study (RECESS). This is a multicenter, randomized, controlled trial in which patients undergoing complex cardiac surgical procedures who are likely to require RBC transfusion will be randomized to receive RBC units stored for either 10 or fewer days or 21 or more days. Randomization will only occur if the blood bank has enough units of RBC of both storage times to meet the crossmatch request; hence, subjects randomized to the 21 day arm will receive RBC of the same storage time as they would have following standard inventory practice of "oldest units out first". The primary outcome is the change in the Multiple Organ Dysfunction Score (MODS), a composite measure of multiorgan dysfunction, by day 7. Secondary outcomes include the change in the MODS by day 28, all-cause mortality, and several composite and single measures of specific organ system function. The estimated total sample size required will be 1434 evaluable subjects (717 per arm).
Trial registration: ClinicalTrials.gov NCT00991341.
(c) 2010. Published by Elsevier Ltd. All rights reserved.
References
-
- Steiner M, Stowell C. Does red blood cell storage affect clinical outcome? When in doubt, do the experiment. Transfusion. 2009;49:1286–90. - PubMed
-
- Hess J. Red cell changes during storage. Transfus Aph Sci. 2010;43 - PubMed
-
- Somani A, Hebbell R. The microcirculation and RBC transfusion. Transfus Aph Sci. 2010;43
-
- Sakr Y. Techniques to assess tissue oxygenation in the clinical setting. Transfus Aph Sci. 2010;43 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL072346/HL/NHLBI NIH HHS/United States
- U01 HL072289/HL/NHLBI NIH HHS/United States
- U01 HL072291/HL/NHLBI NIH HHS/United States
- U01 HL072290/HL/NHLBI NIH HHS/United States
- U01 HL072274/HL/NHLBI NIH HHS/United States
- U01 HL072331/HL/NHLBI NIH HHS/United States
- U01 HL072359/HL/NHLBI NIH HHS/United States
- U01 HL072248/HL/NHLBI NIH HHS/United States
- U01 HL072191/HL/NHLBI NIH HHS/United States
- U01 HL072033/HL/NHLBI NIH HHS/United States
- U01 HL072196/HL/NHLBI NIH HHS/United States
- U01 HL072268/HL/NHLBI NIH HHS/United States
- U01 HL072305/HL/NHLBI NIH HHS/United States
- U01 HL072028/HL/NHLBI NIH HHS/United States
- U01 HL072283/HL/NHLBI NIH HHS/United States
- U01 HL072299/HL/NHLBI NIH HHS/United States
- U01 HL072355/HL/NHLBI NIH HHS/United States
- U01 HL072072/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical